
Original Data and True Copies
Excerpt from the GMP Compliance Adviser, Chapter 15.B.4.4
6 min. reading time | by Christine Oechslein, PhD, and Cornelia Wawretschek
Published in LOGFILE 47/2021
For almost all purposes in the GMP sector, it is mandatory to use original data and documents or only true copies thereof. This requirement also forms part of the ALCOA principle. Since it is only possible in a few cases in operational practice to work with the sole original document, the question arises as to what requirements are to be placed on the requisite true copies (see definition in Figure 15.B-6). Figure 15.B-7 compares different types of copies with the concept of original data.

Figure 15.B-6 Definition of true copy excerpted from the glossary of the WHO Guidance on good data and record management practices (TRS No. 996, Annex 5, 2016)
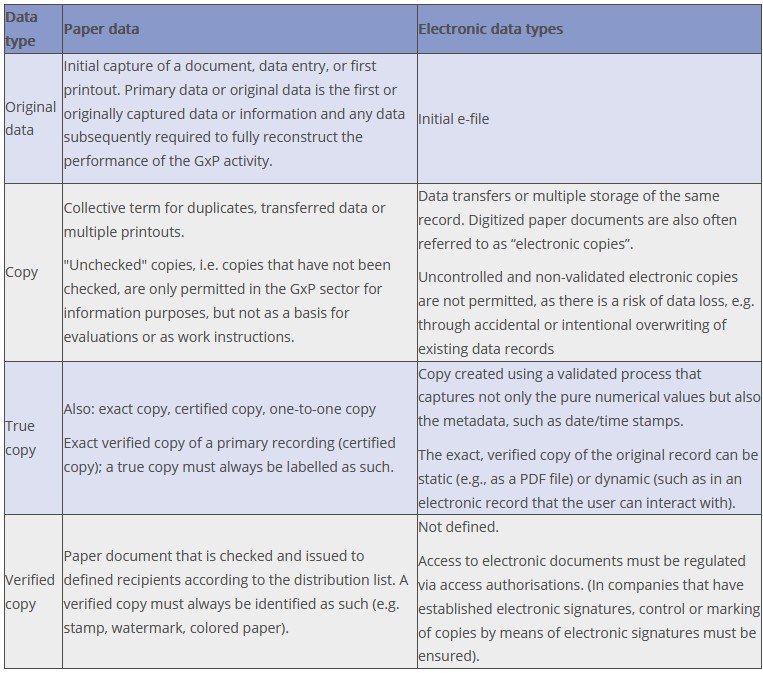
Figure 15.B-7 Comparison of terms used for copies and original data
To avoid mistaking them for the original documents, copies must be marked (authorised copy, verified copy) and signed and dated by the originator. In the past, companies were often required to use only blue pens for signatures or entries in order to be able to distinguish originals from copies. In the age of modern color copiers, this requirement has become obsolete and has no legal basis.
If copies consist of several pages, it is advisable to either permanently bind them together, e.g. with a rivet, and mark only the first page, or to label each page accordingly (page ... of ...).
Thermal paper is used less and less often and is unsuitable for storage because it is not document-proof. This must be taken into account if, for example, chart recorder printouts are produced on this type of paper. In this case, it is recommended to make a copy on normal paper. The copy and the original with the initials or signature must be attached to the documentation.
Small-format attachments (e.g., labels, weighing records) must be pasted and dated and signed off “over the edge” to protect them from loss, i.e., half of the writing on the pasted slip, the other half on the backing sheet.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








