
What’s new for ICH Q9 and ICH Q12?
A report from the 2021 PDA/FDA Joint Regulatory Conference
7 min. reading time | by Sabine Paris, PhD
Published in LOGFILE 38/2021
The PDA/FDA Joint Regulatory Conference, which is now in its 30th year, took place as a virtual event from September 27 – September 29. The Conference focused on the role of effective quality systems in ensuring an ongoing state of control throughout the product lifecycle by vigilantly managing risks to manufacturing and quality.
The session ICH’s Initiatives to Advance Global Harmonization, Innovation, and Continual Improvement Throughout Lifecycle highlighted the revision of ICH Q9 and the implementation of ICH Q12.
Kevin O’Donnell, Market Compliance Manager from the Irish authority HPRA, gave an update on the ongoing revision of ICH Q9 on Quality Risk Management (QRM).
“QRM is a fundamental enabler for the Pharmaceutical Quality System,“ he opened his presentation. The initial discussions for a revision of ICH Q9 started in 2018. HPRA, EMA, an U.S. FDA colleague and an industry representative were involved and prepared the first proposal. The European Commision made a formal proposal to ICH for a revision in 2019. By November 2019, ICH made a decision to proceed with this suggested revision. The finalisation of the revised guideline is currently anticipated for May 2022.

The revision concerns 6 specific topics:
- Subjectivity in QRM
- Product Availability Risks
- Formality in QRM
- Risk-based Decision Making
- Risk Review
- Hazard Identification
These topics will be addressed by adding new guidance that an by developing special training materials. Only for „Risk Review“ ICH Q9’s guidance is sufficient, but training materials will be developed, too.
“This is a very targeted revision of ICH Q9 – it is not a full rewrite. Most of the existing guidance will remain unchanged.“
Scientific approaches to QRM are also stressed in the Concept Paper for the revision. Another area of focus are new technologies and innovation in so far that the revision may support digitisation and emerging technologies, automation, and use of big data, PAT. Furthermore, quality defect issues are mentioned three times in the Concept Paper. This reflects that there are still unmitigated risks as a result of quality defects.
“Why do we need ICH Q12?“ Frank Montgomery from AstraZeneca asked in his enlightening talk about the implementation of ICH Q12 on pharmaceutical product lifecycle management. The need for ICH Q12 results from acceleration of clinical development. The CMC development goes beyond submission. The final supply chain or optimal devices are often introduced post-approval. Thus, the number of post-approval changes (PACs) has also been accelerated. The company’s portfolios are much more diverse than in former days. Another trigger for developing ICH Q12 is the diverse global regulatory environment for PACs. The total expected approval lead time for a world wide approval may even take up to 4 years!
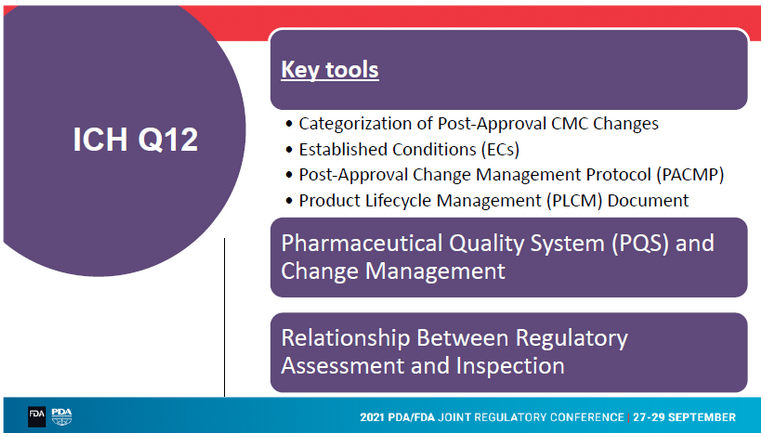
AstraZeneca has already done a succesful ICH Q12 pilot submission in the U.S. for an oral product. They introduced established conditions (ECs) for the synthetic drug substance. The biopharmaceutical risk and impact to the drug product from changes to API were low. The benefits of introducing the tools ECs and PLCM were:
- Site change for drug substance was enabled by agreement of ECs and agreement on necessary supporting stability. Confirmatory stability data were provided rather than in application. The change could be implemented 6 month earlier than usually.
- Serveral process changes progressed without immediate reporting.
- Potential to simplify process via PQS.
- Potential simplification of addition of starting material manufacturers.
Exceptional circumstances call for exceptional action! Frank Montgomery reported that for AstraZeneca’s COVID vaccine the approval for a new drug substance site was approved in 7 calendar days!
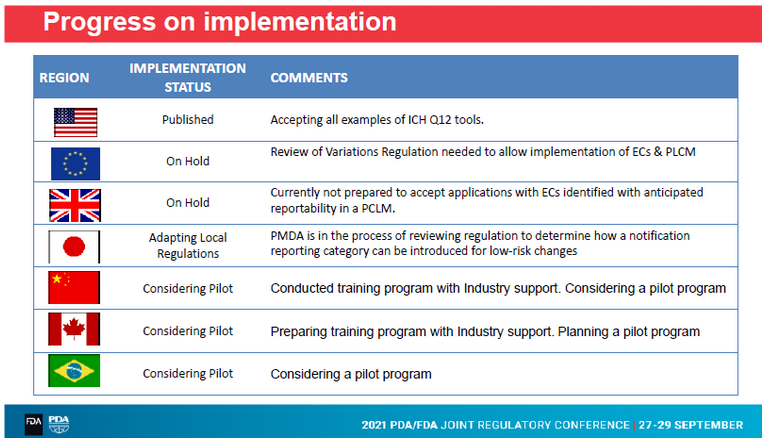
A look at the worldwide progress on implementation of ICH Q12 revealed that up to now only the U.S. has published a national implementation document. All other regions are still in the middle of the process or are considering a pilot progam.
Editor's note:
The European Commission (EC) emphasized that the already existing legal framework always takes precedence over technical and scientific guidelines. Some of ICH Q12’s tools and concepts are not foreseen in the EU legal framework (e.g. definition of ECs, PLCM). They will be considered when this framework will be reviewed. However, in the meantime, the EC is working on the implementation of ICH Q12 within the existing framework.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








