
Water Qualities – Water for Injection (WFI)
Excerpt from the GMP Compliance Adviser, Chapter 5.A.5: Water for Injection (WFI)
5 min. reading time | by Fritz Röder
Published in LOGFILE 12/2025
Pharmaceutical water plays a distinctive and vital role in the pharmaceutical industry. Unlike raw materials and excipients, which undergo quality control and approval before use, pharmaceutical water is used immediately after production. For this reason, it is considered extremely critical. Three types of water are particularly important: potable source water, purified water (PW) and water for injection (WFI). In this feature we focus on WFI. Two previous LOGFILES have already reported on drinking water as source water and on PW.
Stay up to date on water quality by exploring Chapter 5.A, "Water Qualities", in your GMP Compliance Adviser, the world's most comprehensive online GMP knowledge portal.
Production and use
The European Pharmacopoeia defines Water for Injections as water that is intended for use in the manufacture of medicines for parenteral administration whose solvent is water (WFI in bulk), or water that is used to dissolve or dilute substances or preparations for parenteral administration (sterilised WFI). An overview of its potential uses is shown in Figure 1.
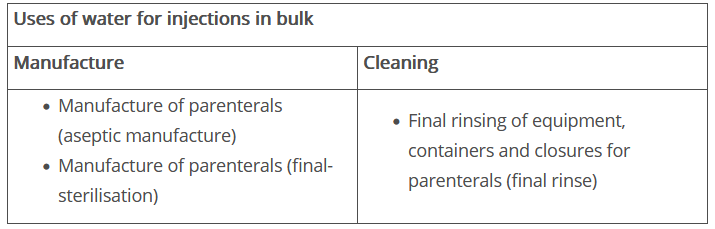
Figure 1 Uses of water for injections in bulk
Water for Injections is obtained from drinking water or treated water, e.g. softened water. In the past, the European Pharmacopoeia permitted the use of distillation only when producing WFI: this changed in 2017. The use of membrane technology as an alternative is now also permitted.
The requirements for cold WFI systems have been the subject of intense discussion in recent years. The ISPE D/A/CH has published a handbook on this subject in German. An international Good Practice Guide has also been published.
Quality requirements
The test parameters and acceptance limits are listed in Figure 2. Testing for heavy metals is no longer required.
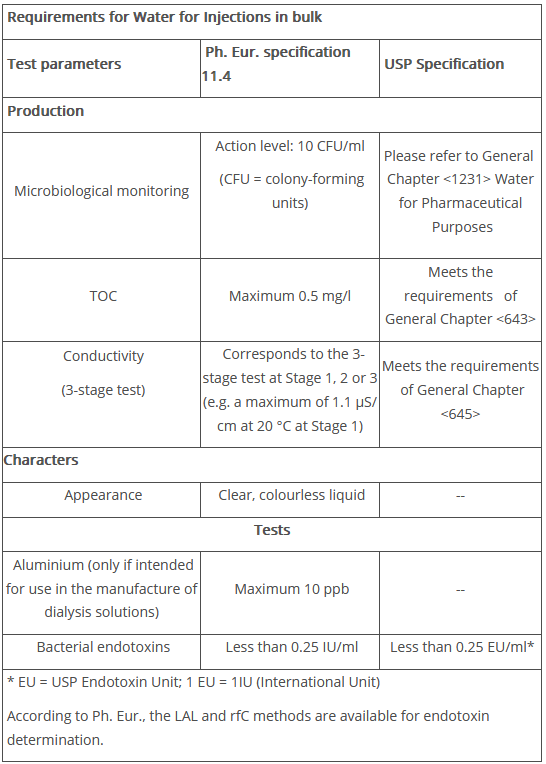
Figure 2 Requirements for Water for Injections in bulk (Ph. Eur. 11.4 and USP 2024 Supplement 12024)
Microbiological monitoring
As is the case in the monographs for Purified Water and Highly Purified Water, precise specifications for microbiological monitoring are included with regard to the test microorganisms for culture media tests, the incubation conditions and the agar to be used. The European Pharmacopoeia does not stipulate testing for specified microorganisms, but it is recommended that exclusion germs are defined for detection. Typically, this is defined as the absence of P. aeruginosa in 100 ml. However, depending on the dosage form manufactured, other exclusion germs may be defined. Under normal conditions, the European Pharmacopoeia only stipulates a total number of colony-forming units of 10 CFU per 100 ml as an action limit. According to the monograph, at least 200 ml of water should be "used" for this purpose. For WFI, this should be interpreted as the amount for filtration.
The incubation period is at least 5 days at 30–35°C. For further details on culture medium and cultivation, refer directly to the European Monograph. The volume to be filtered according to USP <1231> is also 200 ml for WFI.
Determining conductivity (3-stage test)
The measurements can be carried out in-line or off-line. However, there are certain requirements that must be met, the technical specifications and requirements regarding the equipment and implementation, in particular. In practice, in-line measurement has established itself over decades as the better method, but it only covers stage 1 of the test procedure. If the limit of stage 1 is exceeded during in-line measurement, off-line measurement is required.
The 3-stage test in accordance with Ph. Eur. is identical to the specifications in USP <645>. The current pharmacopoeia should be used as a reference if further information is required.
- The conductivity is measured without temperature compensation during stage 1 and the temperature is recorded separately. The measured conductivity value is compared with values in the table for temperature and conductivity in the pharmacopoeia (see Figure 3). If the measured value corresponds to the target value in the table, the test has been passed.
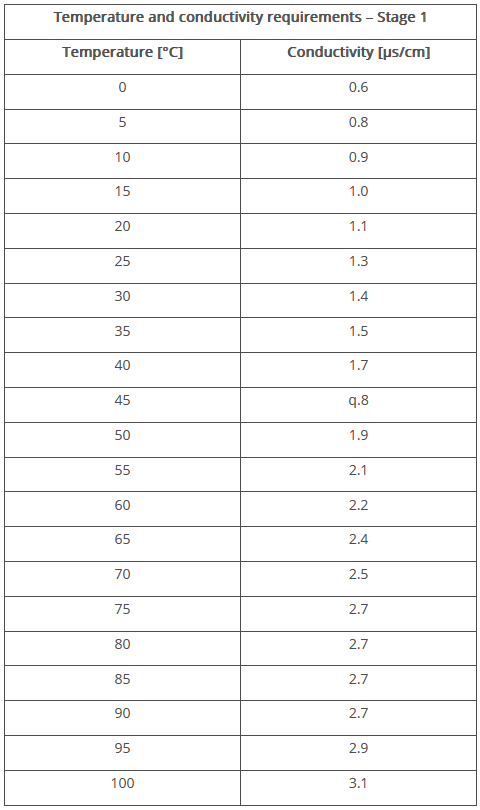
Figure 3 Temperature and conductivity requirements for WFI – Stage 1 (Ph. Eur. 10.6)
- If the measured conductivity exceeds the target value, stage 2 of the test is carried out. The sample is heated to 25 °C and stirred until a constant conductivity is reached. The measured value must not exceed 2.1 µs/cm.
- If stage 2 is not successful, stage 3 of the test must be carried out immediately. The solution is again tempered to 25 °C and the pH value is determined with an accuracy of 0.1 after the addition of fresh potassium chloride solution. Using the table in the pharmacopoeia (see Figure 4), the conductivity limit value for the measured pH value is determined and compared with the conductivity value measured during stage 2. The substance meets the requirements if the conductivity value measured during stage 2 does not exceed the limit value. If a greater value is measured or the pH value is outside the permitted range (5.0 to 7.0), the sample does not meet the requirements.
The description of conductivity measurement also contains precise specifications for the conductivity cell (material, cell constant) and information on measurement accuracy and calibration of the conductometer.
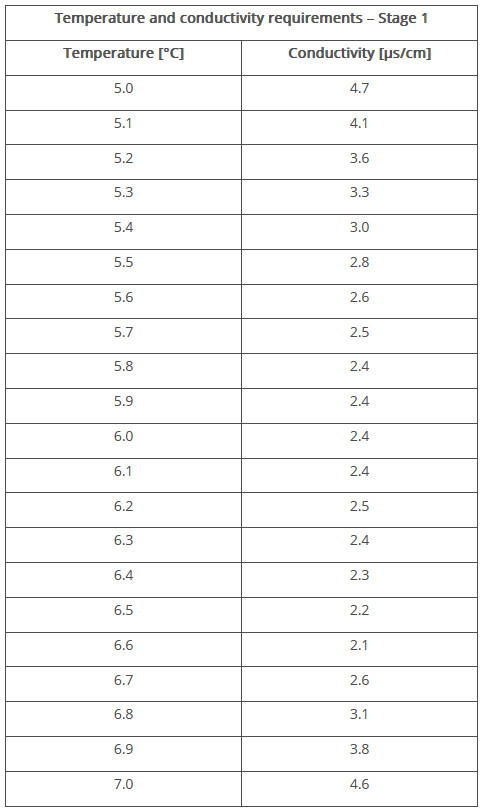
Figure 4 Temperature and conductivity requirements for WFI – Stage 3 (Ph. Eur. 10.6)
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








