
Water Qualities – Purified Water
Excerpt from the GMP Compliance Adviser, Chapter 5.A.3: Purified water
5 min. reading time | by Fritz Röder
Published in LOGFILE 09/2025
Pharmaceutical water occupies a unique position within the pharmaceutical industry. Whereas raw materials and excipients are used only after quality control and approval, pharmaceutical water is used immediately after production. For this reason, it is considered extremely critical. Three types of water are of particular importance: potable source water, purified water (PW) and water for injection (WFI). In this issue we will focus on PW, while a future issue of LOGFILE will cover WFI.
Stay up to date on water quality by exploring Chapter 5.A: Water Qualities in your GMP Compliance Adviser, the world's most comprehensive online GMP knowledge portal.
Production and use
Demineralised water, deionised water, pure water or aqua purificata are commonly used for purified water. When choosing a process, technologies such as reverse osmosis (RO) are described, but in principle the pharmaceutical manufacturer is free to choose.
Because the requirements (in Germany) for microbiological purity of purified water are no stricter than those that apply to drinking water (max. 100 CFU/ml), simple ion exchanger systems are still used, especially in very large facilities. Modern manufacturing processes, however, normally use membrane technology for desalination. Depending on the initial salt content, a second membrane stage or downstream electrochemical desalination may be necessary to achieve the required desalination.
In accordance with the Pharmacopoeia, purified water in bulk can be used during the manufacture of preparations that do not have to be sterile or free of pyrogens. Tablets, capsules, ointments, lotions or nasal sprays are typical dosage forms. Purified water is often used as a starting material for the manufacture of water for injections.
In accordance with the Pharmacopoeia, purified water in bulk can be used during the manufacture of preparations that do not have to be sterile or free of pyrogens. Tablets, capsules, ointments, lotions or nasal sprays are typical dosage forms. Purified water is often used as a starting material for the manufacture of water for injections.
Quality requirements
The European Pharmacopoeia (Ph. Eur.) and the United States Pharmacopeia (USP) both stipulate the test parameters and acceptance limits shown in Figure 1. Limit values can always be found directly in the monographs in the European Pharmacopoeia. The United States Pharmacopeia, however, refers the reader to the relevant General Chapter.
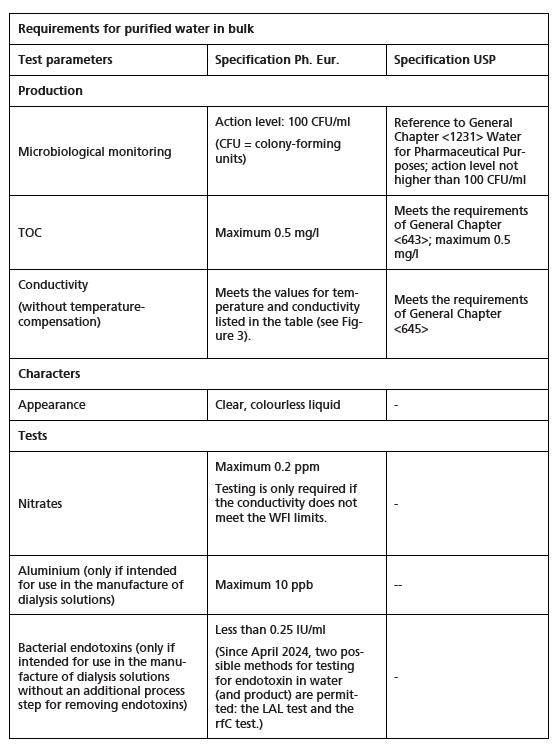
Figure 1 Requirements for purified water in bulk
Microbiological monitoring
The specified action level for purified water in the European Pharmacopoeia is 100 CFU/ml. In practice, however, this limit appears to be understood as a specification rather than an action limit. The monograph also includes precise information on:
- The performance of the test (maximum membrane filtration of 0.45 µm)
- The agar to be used (only Ph. Eur. R2A agar)
- The incubation conditions (a minimum of 5 days at 30 to 35 °C)
- The test bacteria to be used for the culture media tests
These incubation conditions were chosen at the time as a compromise between incubation period (business risk) and recovery (quality risk). This compromise has also proven itself in practice. Drinking water germs are incubated at two temperatures (22 °C and 36 °C), germs in ultrapure water only at one temperature (30–35 °C). An incubation period of more than five days would cause problems in production, because many manufacturers already produce their bulk or finished products "at risk" until the water results are available. Longer incubation times would only further increase the economic risks in manufacturing.
The European Pharmacopoeia specifies a "suitable" volume as the volume to be filtered. In contrast to WFI, only small volumes can be filtered here to obtain results that can still be counted.
The United States Pharmacopeia contains recommendations on the general implementation of the incubation (see USP <1231>); however, the expectation is that the pharmaceutical manufacturer specifies the absence of defined microorganisms based on a risk assessment and designs a suitable method of incubation. The volume to be filtered is specified as "up to 100 ml".
Neither pharmacopoeia explicitly claims for testing for specified microorganisms. However, it is practically impossible to escape an observation in the course of a regulatory inspection without providing information on particular bacteria types. Proof of the absence of Ps. aeruginosa and E. coli in 100 ml is often expected by the authorities ("exclusion germs"). This requirement makes sense, but it should be noted that it is not stipulated in the regulations.
For drinking water E. coli is already defined as an exclusion germ. Thus, one could expect (in case of a negative result) that the feed water of the PW system is free of E. coli. Nevertheless, it may be useful to test purified water for E. coli at production tapping points to monitor the general hygiene status in the manufacturing plant. Positive findings at sampling points indicate improper handling or inadequate industrial hygiene, but not a biofilm in the water system. Of the species listed in Ph. Eur. 2.6.13 and USP <62>, Pseudomonas aeruginosa is the only one that is viable in ultrapure water and is therefore preferred in practice.
For certain applications and depending on the quality of water treatment, additional measures to reduce the microbial count may be necessary when storing purified water. If purified water is used as autoclave cooling water in direct contact with the containers, significantly stricter limit values should be established. Guidance on this can be found in the EMA Guideline on the sterilisation of the medicinal product, active substance, excipient and primary container, which specifies requirements depending on the application.
Determining conductivity
Both pharmacopoeias also contain a detailed description of conductivity measurement (Ph. Eur. 2.2.38, USP <645>). Measuring devices with defined technical requirements are specified for determining conductivity (on-line or off-line). The cell constant of the conductivity cell must be certified by the supplier and regularly calibrated using a certified reference solution or a conductivity cell with a certified cell constant. Additional requirements include system calibration with an accuracy of ±2 % (USP <645>) and calibration of the conductometer. The maximum deviation permitted during temperature measurement is ±2 °C. The European water quality requirements are considered met when the values listed in Figure 2 are not exceeded at the given temperatures.
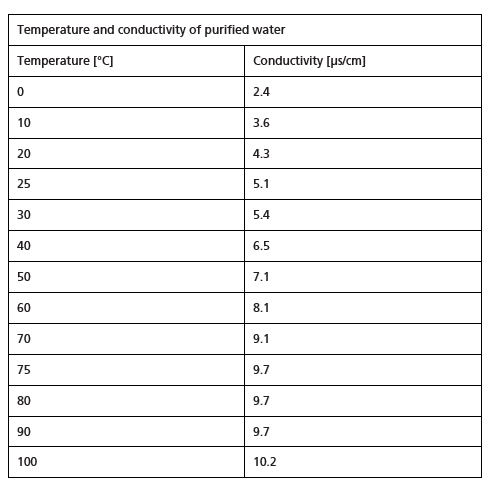
Figure 2 Temperature and conductivity of purified water (European Pharmacopoeia, 10.6, 2021)
Elemental impurities
If the conductivity of the purified water does not correspond to the equivalent requirements for Water for Injections (i.e. 1.3 µS/cm at 25°C), a risk assessment according to general chapter 5.20 Elemental impurities is carried out. The risk assessment should consider the role of water in the manufacturing process, when water is used in a process but is no longer present in the final product.
This requirement was introduced in Ph. Eur. 9.4 and replaces the former test for heavy metals.
Purified water in containers
Purified water that has been filled into containers for dispensing is a final product. Additional purity testing must be carried out. The specifications in the "Purified water in containers" pharmacopoeia monograph then apply. This product may not contain additives and must pass the purity tests for "Purified water in bulk" and the following tests:
- Acidity or alkalinity
- Oxidisable substances
- Chlorides
- Sulfates
- Ammonium
- Calcium and magnesium
- Residue on evaporation
- Microbial contamination
In addition, the liquid should be clear and colourless.
The microbial purity is specified at 100 CFU/ml. It is determined using casein soya bean digest agar.
The USP also specifies sterile purified water. This water must meet the specifications for sterility in addition to the chemical/physical parameters.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








