
Water Qualities – Drinking Water as Source Water
Excerpt from the GMP Compliance Adviser, Chapter 5.A.2: Drinking water as source water
6 min. reading time | by Fritz Röder
Published in LOGFILE 07/2025
Pharmaceutical water has a unique position in pharmaceutical industry. As raw materials and excipients are only used after quality control and subsequent release, pharmaceutical water is used immediately after production. For this reason, pharmaceutical water is considered extremely critical. Three types of water are of particular interest: Drinking water as source water, purified water (PW) and water for injections (WFI). In today’s feature we report on drinking water as source water. In future LOGFILES, we will report on PW and WFI.
Keep up to date with Water qualities by taking a closer look at chapter 5.A: Water Qualities in your GMP Compliance Adviser, the most comprehensive online GMP knowledge portal worldwide.
Pharmaceutical water occupies a unique position in the pharmaceutical industry. Raw materials and excipients are normally only used after quality control and subsequent release. Pharmaceutical water, however, is always used immediately after it has been produced because microbiological approval can take several days. For this reason, pharmaceutical water is regarded as being extremely critical, and there are many specifications in the pharmacopoeias and GMP regulations.Three water types are of particular interest:
- Drinking water as source water
- Purified water (PW)
- Water for injections (WFI)
In addition, until 2019, the European Pharmacopoeia included the water quality "highly purified water (HPW)", which has been deleted from the pharmacopoeia. Nevertheless, such systems still exist in the industry.
Drinking Water as Source Water
In accordance with the regulations, drinking water is the source water, i.e. the raw product used to produce other water qualities. Drinking water is, therefore, a raw material and as such must undergo an incoming goods inspection. Waterworks analyses are often the only proof available for the quality of drinking water. In general, however, additional on-site monitoring is expected as the samples for these analyses are taken by the utilities at their waterworks or elevated tanks. The distance that drinking water travels from the elevated tank to the point of entry into an ultrapure water system can be several kilometres. Drinking water can be contaminated along the way. In addition, the supplier's responsibility ends at the pharmaceutical company's transfer point. In the case of the monitoring mentioned above, microbiology is usually the main concern. However, other parameters may be added. For example, the WHO guidelines (Figure 1) state:

Figure 1 Excerpts from ‘Annex 2 WHO good manufacturing practices: water for pharmaceutical use’ for the production of drinking water.
Accordingly, a drinking water network is not subject to qualification. The current version of the German Drinking Water Ordinance of 2023 explicitly states that systems for the distribution of drinking water must be planned, constructed and operated at least in accordance with the generally recognised rules of technology (§13). Accordingly, suitable piping materials must be always used and appropriate safety devices (e.g. backflow preventers according to EN 1717) installed when building new facilities.
It is also important to know the source of the drinking water because there are major differences between surface water and good-quality well water. The main differences between the two sources are listed below.
Ground water
Water from the depths of the earth, well filtered, consistent quality:
- Low turbidity
- Constantly low temperatures
- Generally high salt content, geogenic
- No organic contamination
- No anthropogenic impurities
- Only treated if necessary
- No parameter fluctuation
- Mostly a low bioburden
Surface water
Water from the surface of the earth, rivers, lakes, etc.:
- High turbidity, high colloid index
- Temperature differences in summer/winter
- Parameter fluctuation possible
- Contaminated with different substances
- Can contain humic substances
- Often burdened with organic substances
- Often microbiologically burdened, high TOC
If you have the choice between these two qualities of drinking water, you should always choose groundwater as the starting quality, as it is much less variable. As a result, a pure water system is much easier to operate.
Quality requirements
Drinking water is water that meets the statutory quality requirements for human consumption and use. However, the water can contain substances that have to be removed prior to the generation of pharmaceutical water. Typical examples are the hardness of water or its organic contamination. Water softeners are normally used to treat hard water. Organic contamination, however, is often only noticed when the system membrane is blocked, and fluctuations are possible at any time. When systems that use modern membrane technology are used for cold WFI production, this parameter has particular significance and requires proper monitoring and documentation.
The quality of drinking water in Germany is defined in the German Drinking Water Ordinance. The permissible limits for substances found in drinking water and specifications on the scope, frequency and implementation of testing are outlined in the appendices of the Drinking Water Ordinance. Comparison of the in-house requirements with the latest version of the Drinking Water Ordinance (or other national regulations, as applicable) on a regular basis is recommended to ensure that they meet the requirements.
The microbiological quality of the drinking water is an important parameter regarding pharmaceutical use.
- Escherichia coli and enterococci (faecal streptococci) must not be detectable in 100 ml water.
- Pseudomonas aeruginosa and legionella, on the other hand, may be present in drinking water in small numbers
The Ordinance lists the total microbial count as an indicator parameter and the colony count must not change in any significant way. The Drinking Water Ordinance, however, also stipulates a limit of 100 CFU/ml for the water at the point of use of the consumer. This limit should be the feed water action limit for manufacturers of medicinal products.
There are also limit values and indicator parameters for heavy metals, organic compounds and other physical parameters, e.g. electric conductivity. For detailed information, please refer to Annex 2 and 3 of the Drinking Water Ordinance. In practice, checking the respective critical values and/or fluctuating chemical and physical parameters of the drinking water before use can suffice. These parameters can include nitrate and organic contamination, for example.
From April 2024, it will no longer be necessary to test ultrapure water for nitrate in most cases. However, elevated nitrate levels in drinking water can be an indicator of pesticide use in agriculture (depending on the season).
In addition to supplying the pharmaceutical water treatment system, drinking water is also used for pre-rinsing the equipment and containers during cleaning cycles. However, if drinking water is too hard, it may not be suitable (or a water softener is connected upstream). Drinking water can also be used during the initial stages of active substance manufacture (Figure 2). The EMA Guideline on the quality of water for pharmaceutical use contains further details.
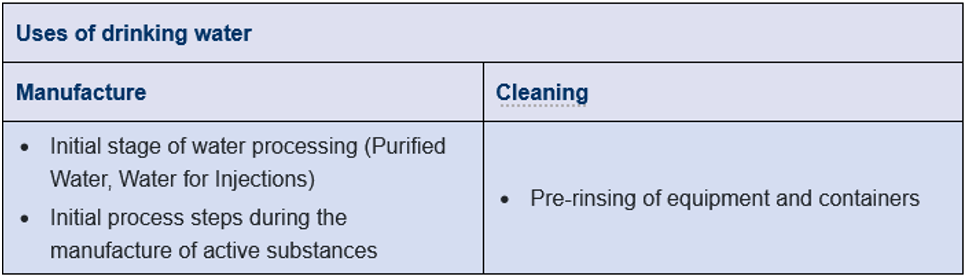
Figure 2 Uses of drinking water in the pharmaceutical industry.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








