
Typical GMP Deficiencies at Manufacturers of Chemical APIs - An Overview
Excerpt from the GMP Compliance Adviser, Chapter 20.F.5.14
7 min. reading time | by Norbert Waldöfner, PhD
Published in LOGFILE 41/2021
Figure 20.F-24 provides an overview of the GMP deficiencies that, in the author's experience, are frequently encountered during audits of active substance manufacturers.
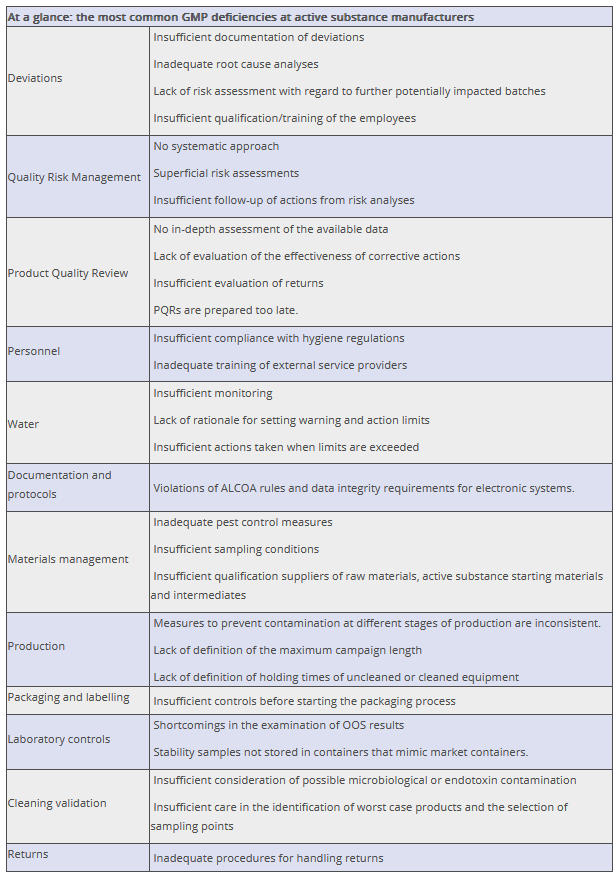
Figure 20.F-24 Common GMP deficiencies at active substance manufacturers
Thematically, there is thus a striking overlap here with the deficiencies found during GMP inspections of medicinal product manufacturers (see 21.C Frequent deficiencies in GMP inspections, their recurring pitfalls and how to avoid them). From the author's point of view, the production of active substances generally takes place at a high level; with regard to the fulfillment of the respective GMP requirements, active substance manufacturers are not necessarily in a worse position than medicinal product manufacturers.
Due to the increasing use of electronic systems, it is to be expected that deficiencies in the area of documentation or, for example, the handling of deviations and OOS results will be identified and avoided more quickly in the future. However, this will also result in new sources of errors and requirements, especially with regard to the validation of electronic systems and the maintenance of data integrity. From the author's point of view, the focus of audits at active ingredient manufacturers will certainly continue to shift in the direction of checking these requirements.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








