
The Draft ICH Q1 Guideline: A Fresh Take on Stability
5 min. reading time | by Joachim Ermer, PhD
Published in LOGFILE 16/2025
How does the draft ICH Q1 guideline affect the stability study design and conduct of drug products and drug substances? Joachim Ermer, PhD, summarises the most important changes and comments on critical aspects.
A comprehensive discussion of all planned changes and innovations can soon be found in the GMP Compliance Adviser under the heading ‘Current Topics’.
Stability testing is an essential part of drug development and approval. Its purpose is to ensure the quality, safety and efficacy of a drug over a defined period of time, referred to as the shelf life. Its importance is also emphasised by the fact that the stability guideline Q1A Stability Testing of New Drug Substances and Products was the first topic addressed in the international harmonisation process in October 1993.
Further aspects of stability testing were addressed in subsequent guidelines:
- ICH Q5C: Quality of Biotechnological Products – Stability Testing of Biotechnological/Biological Products (Nov 1995)
- ICH Q1B: Stability Testing: Photostability Testing of New Drug Substances and Products (Nov 1996)
- ICH Q1C: Stability Testing for New Dosage Forms (Nov 1996)
- ICH Q1D: Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products (Feb 2002)
- ICH Q1E: Evaluation of Stability Data (Feb 2003)
- ICH Q1F: Stability Data in Climatic Zones III and IV (June 2006, withdrawn)
In the 2000s significant developments occurred in the field of pharmaceutical manufacturing, particularly with the introduction of the ICH Q8 Pharmaceutical Development Guideline and the application of Quality by Design (QbD) principles and tools, and the ICH Q9 Quality Risk Management (QRM) Guideline. The focus was on taking a holistic view of the entire lifecycle, which resulted in FDA and EU guidelines on process validation. These principles were also reflected in further ICH guidelines, such as the ICH Q12 Lifecycle Management Guideline which, for the first time, included analytics and quality control. Other examples were in the revised ICH Q2 Analytical Validation Guideline and the development of a new ICH Q14 Analytical Procedure Development Guideline.
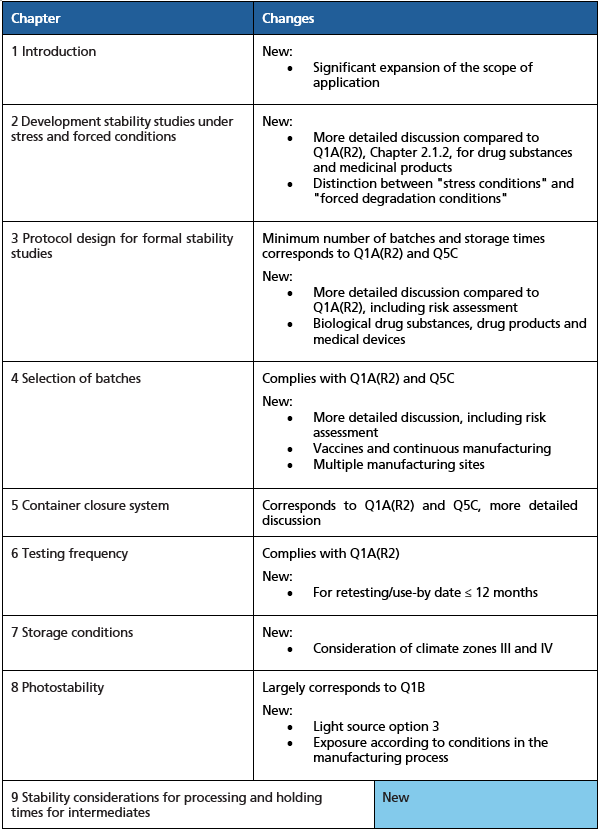
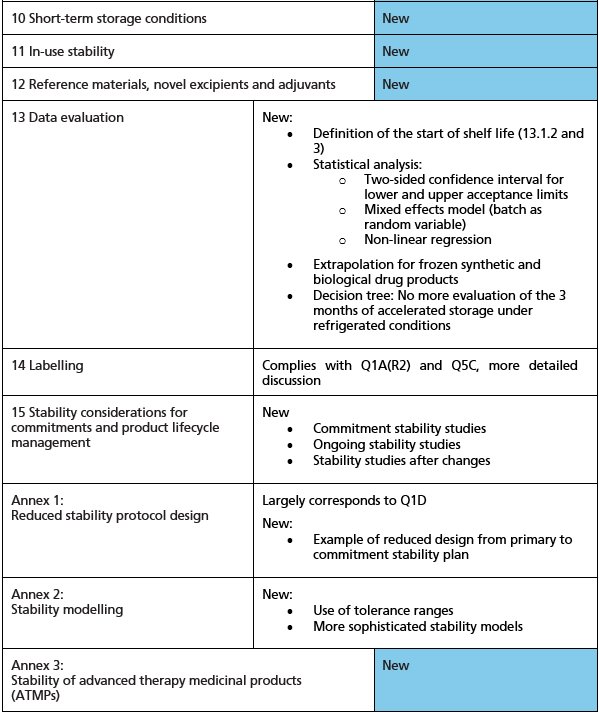
Consequently, the decision was made in 2022 to revise the ICH Stability Testing Guideline. In addition to taking the aforementioned principles into account, a key objective was to consolidate the various guidelines for drug products containing chemical and biological drug substances. Despite the significant expansion of the ICH scope making this complex task more challenging, the ICH expert group has managed to adhere to the 2022 sched-ule, publishing the draft guideline for public consultation in April 2025. Following, the author outlines the additions he considers important, as well as some shortcomings and areas for improvement in the ICH Q1 draft guideline. Figure 1 provides an overview of the guideline's key changes and reference.
Figure 1: Overview of the main changes in ICH Q1
Summary
The application of risk assessment principles to all decisions runs throughout the entire Q1 draft guideline, and the corresponding justifications are expected to follow suit. The guideline has also been consistently aligned with the lifecycle approach, which is why it is explicitly required to be considered in its entirety.
The draft allows greater flexibility than the previous guidelines for the following aspects:
- Reduced storage times if there are no changes to the critical quality attributes (CQAs) or alternatives (modelling)
- Photostability: Option 3: Fluorescent or LED lamp with ambient light conditions (> 400 nm) during manufacture, processing and application
- Extrapolation for frozen stored synthetic substances
- Extrapolation for frozen stored biological substances
- Annex 1: Reduced stability protocols
In the author's opinion, some aspects require further discussion or should be supplemented with examples for clarification:
- Statistical analysis: Application of the tolerance range approach (broader than confidence intervals) and non-linear regression
- Higher stability modelling: The comment "Not intended to replace long-term stability studies" contradicts other discussions ("Greater extrapolation may be possible with other modelling methods (Annex 2 – Stability modelling)").
- Decision tree: "The data show little or no change and little or no variability." How much is "little" or "none"? No variability is not scientifically sound!
In the author’s opinion the following points should be corrected or supplemented:
- Statistical analysis: For CQAs with upper and lower acceptance criteria and a known direction of change (prior knowledge), the one-sided confidence interval limit is scientifically justified.
The statistical analysis should only be performed if there is a significant increase, as extrapolation of the confidence intervals only makes sense if there is a (true) change. - Decision tree scenario B: A significant change under accelerated conditions is not permissible.
- Extrapolation: Extrapolation for frozen stored synthetic substances is scientifically justified in the absence of or with minor changes and should be taken into account in the decision tree.
The author’s conclusion:
Given the complex nature of the topic and the numerous ICH stakeholders involved, a good balance has been achieved between continuity, further development and modernisation. The need for corrections and additions is very limited. However, to further enhance the Q1 guideline’s practicality, I would like to encourage you to comment on any crucial aspects from your side while the document is under public consultation.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








