
Sterile Filtration (PUPSIT)
Excerpt from the GMP Compliance Adviser, Chapter 12.A.9
5 min. reading time | by Xenia Dimont
Published in LOGFILE 18/2024
The term "PUPSIT", which stands for the Pre-Use Post-Sterilization Integrity Test, has been included in the revised Annex 1. While the term is new, the requirement to check the integrity of a sterilizing filter after it has been sterilized and before it is used for sterile filtration is not new. This has been included in Annex 1 since 1998.
The background to this requirement and the explanation of its implementation were included in the EMA's Q&A section "Guidance on good manufacturing practice and good distribution practice: Questions and answers" already in 2007. In the Annex 1 version of 2008, the requirement was listed under No. 113.
| EU GMP Guideline Annexes: Supplementary requirements: Annex 1: Manufacture of sterile medicinal products: |
|
1. How should the integrity of sterilizing filters be verified? H+V June 2007 Annex 1, paragraph 85 states, 'the integrity of the sterilized filter should be verified before use and should be confirmed immediately after use by an appropriate method such as a bubble-point, diffusive-flow or pressure-hold test'. The filter-sterilization process may be physically stressful for the filter. For example, high temperatures during the process may cause the filter to distort, potentially leading to fluid pathways that allow the passage of particles greater than 0.2 µm in size. The performance of a filter can improve with use, as particles begin to block individual pathways and remove larger pathways that smaller particles could successfully navigate. For these reasons, filters should be tested both before use but after sterilization and again after use. Furthermore, testing should be performed in situ in order to verify the integrity of the filter complete with its housing. |
What purpose does the PUPSIT serve?
The PUPSIT is performed to test the integrity of a sterilization filter and associated components after the filter has been sterilized but before it is used for sterile filtration. The test is designed to identify potential masking effects. The masking effect is a phenomenon in which a defect in a sterilization filter becomes clogged during the filtration process, for example by contaminants and/or components, resulting in a successfully passed integrity test after the filter has been used. A test after sterilization and before use of the filter should make it possible to detect such defects before using the filter and eliminate this risk.
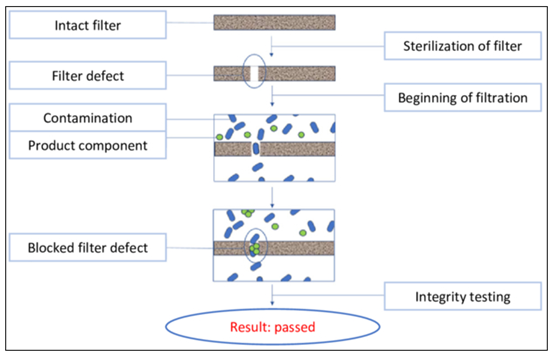
Possible methods for testing the integrity of sterile filters are listed in the revised Annex 1 as examples such as the bubble test, water intrusion test, diffusion flow and pressure hold tests.
Why are the PUPSIT requirements so controversial?
The necessity, practicability and risk-benefit relation of PUPSIT were controversially discussed during the revision of Annex 1. Among other things, concerns were expressed by experts and industry associations that the implementation of PUPSIT would entail additional risks, such as further interventions in the aseptic area, increased complexity of the filtration system, possible failure of PUPSIT components and stress on the sterilized filter during the test procedure. The probability of microbiological contamination due to a masking effect is considered to be very low.
As a result, the risk of contamination or impairment of product quality due to PUPSIT would outweigh the risk of product contamination due to a filter defect that is concealed during use of the filter and not detected afterwards.
Last but not least, PUPSIT is a costly and time-consuming measure, the cost-effectiveness of which is being critically scrutinized in terms of the risk-benefit relationship.
For which situations/processes/products is there a real risk?
There is currently little data available on how likely the masking effect is to occur in a specific case and which circumstances enable or favor this. Current findings indicate that the masking effect actually occurs only rarely and depends, among other things, on the extent of the filter defect and the properties of the material to be filtered:
- The filter defect is large enough to allow microbial contamination to pass.
- The filter defect is small enough to remain undetected when clogging occurs.
- A sufficient material or particle load is present to allow non-detectable blockage of defects.
- The product has a tendency to agglomerate.
A general assessment of the masking effect appears difficult, as products and processes and subsequent combinations thereof are generally unique.
The studies "Test Process and Results of Potential Masking of Sterilizing Grade Filters" and "Datamining to Determine the Influence of Fluid Properties on the Integrity Test Values" published in 2020 can provide assistance for an individual assessment. These publications are the results of the Sterile Filtration Quality Risk Management (SFQRM) consortium, which was launched by the Parenteral Drug Association (PDA) and BioPhorum Organizations Group (BioPhorum) at the end of 2017.
Who has to implement PUPSIT?
Although the requirement for PUPSIT was already present in the previous version of Annex 1 – as explained above – it has been significantly modified in the revised Annex 1. While the requirement is still considered mandatory, it is acknowledged that PUPSIT is not always feasible in certain cases due to process constraints (e.g. filtration of small volume products such as radiopharmaceuticals) and in such cases an alternative approach would be permissible under certain conditions.
This means that PUPSIT is generally required for all processes involving sterile filtration of products that cannot be sterilized in their final container. Only in cases where PUPSIT is not possible an alternative approach can be considered.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







