
Stability Studies During the Authorisation Phase of Medicinal Products
Excerpt from the GMP Compliance Adviser, Chapter 14.E.5.3
7 min. reading time | by Heike Meichsner, Olaf Mundszinger, PhD, Susanne Schweizer
Published in LOGFILE 32/2021
The registration batches correspond as nearly as possible to the later commercial batches in the marketing phase with regard to the equipment, starting materials and manufacturing processes used.
The registration batches correspond as nearly as possible to the later commercial batches in the marketing phase with regard to the equipment, starting materials and manufacturing processes used. Storage of the batches takes place in the container/closure system(s) provided later in the marketing phase. These batches are thus representative of the product that will later be available on the market.
The stability data on the so-called registration batches are collected in a standardised manner according to the ICH Q1A(R2) guideline.
The stability program must include long-term studies as well as intermediate and accelerated studies. It must be designed in such a way that analytical, release and shelf-life specifications can be derived from the data. All stability results collected shall be considered for their definition. The test frequency within the stability studies must therefore be designed in such a way that a complete stability profile can be established.
How many batches must be placed on stability?
At least three batches of the same formulation and primary packaging should be stored for authorisation stability. Three different API batches should be used. Two of the three finished medicinal product batches must be at least pilot scale size (1/10 of the subsequent production batch or 100,000 units). The third batch may be smaller, if justifiable, but shall be at least 25,000 units. This applies to each strength and primary packaging unit. The procedure is illustrated in Figure 14.E-8.
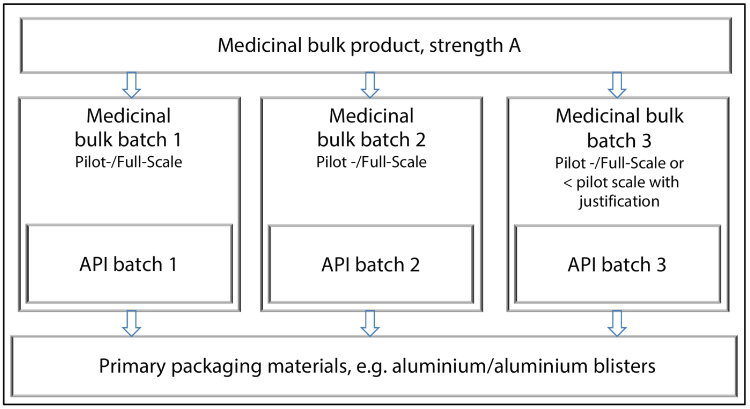
Figure 14.E-8 Exemplary definition of batches to be included in studies
If the dossier includes several dosage strengths, a reduction of the study design can be considered.
How are the storage conditions and testing frequency defined?
For storage conditions and test frequencies, the Q1A(R2) and WHO (Technical Report Series, No. 1010, Annex 10) guidelines are applied (see Figure 14.E-9 and Figure 14.E-10).
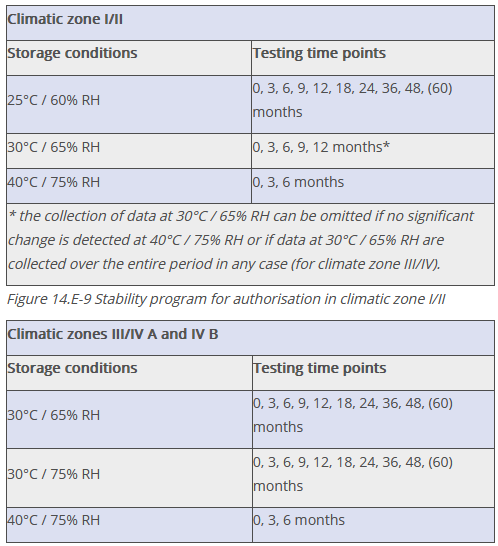
Figure 14.E-10 Stability program for authorisation in climate zones III/IV A and IV B
What insights should the studies provide?
The authorisation stability results are intended to confirm the findings on stability and storage conditions obtained during the development phase. Taking into account the data from the registration batches and using the guideline ICH Q1E Evaluation of Stability Data (E.1.E ICH Q1E: Evaluation of Stability Data) the shelf life and expiry date, the release and shelf life specifications as well as the storage conditions and storage instructions are established.
Furthermore, additional stability studies are to be performed on the formal registration batches:
- Photo-stability testing to determine the influence of light on product quality
- Studies for the determination of the intermediate and bulk hold times
- In-use studies to determine the life of opened product
- Freeze and thaw studies and/or shipment studies to determine any limitations for transport conditions
What data are required for the marketing authorisation application?
In general, stability studies must cover the entire term of the shelf life. This is set at a maximum of five years. Shorter periods – usually 3 years – are frequently targeted. Since it is not possible to wait for the entire test period for approval, minimum requirements have been defined in this respect. Thus, for studies under long-term conditions, results over 12 months have to be submitted, for the accelerated conditions over six months. Therefore, the submission usually takes place before the end of the study program of the registration batches. As part of the marketing authorization application, the applicant must therefore provide a commitment to continue the stability studies until they are completed.
The stability commitment describes precisely the extent to which stability studies started on the registration batches will be continued after receipt of marketing authorisation and/or whether stability studies of the first production batches (follow-up stability studies) are required. The necessity and the extent of the implementation of a follow-up stability is coordinated with the competent authority within the framework of the authorisation procedure.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








