
Sliding doors in cleanrooms - "no go" or "best practice"?
Shortened and edited excerpt from the GMP Compliance Adviser, Chapter 3.E "Cleanroom Construction Components"
5 min. reading time | by Harald Flechl / Doris Borchert (editorial editing)
Published in LOGFILE 35/2020
In cleanrooms, it is necessary to create a clear demarcation to adjacent, less clean areas. Since the beginning of clean room technology, airlocks have established themselves as an effective means of separating rooms.
The doors arranged one behind the other for access to the clean area must not be opened simultaneously in order to maintain the separation of the areas. This arrangement can still be seen as the state of the art in cleanroom technology.
Hinged doors, single or double-leaf with inactive and active leaves, striking plate, hinges and handle sets are the most frequently used doors in cleanrooms. But often undetected risks are concealed here:
Uncontrolled dead spaces
The retractable bottom seal (Figure 1) in hinged door leaves, which is often desired and installed because of the higher sealing tightness, does not meet the requirement for easy accessibility for cleaning. In the door leaf, an uncontrolled dead space results from the use of the seal, which is open at the end faces of the door leaf and allows cleaning only when the door leaf is removed.
A retractable bottom seal (Figure 1) in hinged door leaves is often requested and installed because of the higher sealing tightness, however it is not easy-to-clean. The seal results in an uncontrolled dead space in the door leaf, which is open at the end faces of the door and permits cleaning only when the door leaf is removed.
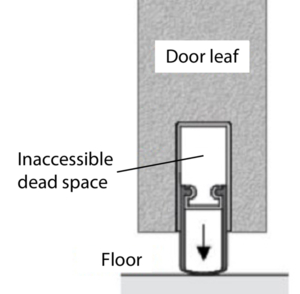
Figure 1 Integrated retractable bottom seal for hinged doors
A further risk is concealed in the locking mechanism of the doors. The fixtures including bolt, latch, door locks and the hinges are often overlooked as weak points. Most doors here have uncontrolled and hard to clean openings (striking plate open towards the entire door frame). Monoblock systems often offer the possibility of integrating door closers into the panel. These are not visible from the outside, but replacement should be possible.
Air turbulences
Manual opening of a hinged door creates a high air turbulence (depending on the opening speed of the door) which causes uncontrolled air exchange between adjacent rooms of different cleanroom classes. In order to enable smooth and steady closing and thus avoid the disadvantages mentioned above, cleanroom doors can be equipped with drives for automatic opening by sensors or by manual control units and also including door closers. The suitability of the drive for use in a clean room should be proven (e.g. suitability test by an independent institute). The safety requirements of the European harmonized product standard EN 13241 must be complied with. Thresholds or stop rails on the floor are not recommended.
Sliding doors as a solution to the problem?
In view of the risks and disadvantages mentioned above, the question inevitably arises as to whether sliding doors are a sensible and GMP-compliant alternative to the hinged doors normally used. With regard to sliding doors, Annex 1 of the EU GMP Guide recommends the following:
„To reduce accumulation of dust and to facilitate cleaning there should be no un-cleanable recesses and a minimum of projecting ledges, shelves, cupboard and equipment. Doors should be designed to avoid those uncleanable recesses; sliding doors may be undesirable for this reason.”
The word selection in the German translation of the text “sliding doors may be undesirable” was somewhat harsher than the original English intent, which in the past has led to a general distaste for sliding doors in GMP environments there, as it was interpreted that “sliding doors are not permitted”. Practical inspection experience has shown that in most cases sliding doors as well as high-speed rollup doors are accepted by the agencies for cleanroom applications.
When installing automatic sliding doors, it should be considered that the drives should be installed on the side with the lower cleanroom class (see view from side with higher cleanroom class in Figure 2).

Figure 2 Automated sliding door with status indicator, emergency stop and cleat (G+H Reinraumtechnik GmbH, D-67063 Ludwigshafen; www.guh-reinraumtechnik.de)
Analogously, the same recommendations apply for so-called high-speed doors (Figure 3), which roll up in the vertical direction to open and close.

Figure 3 High-speed door with electrical drive - clean room certification from Fraunhofer Institute (source: ASSA ABLOY Entrance Systems, www.assaabloyentrance.de)
Horizontal sliding doors and vertical rolling high-speed doors provide a number of decisive advantages in cleanrooms:
- They have low space requirements.
- As a result of their roll-down design, they provide effective seals on all four sides.
- Undesirable strong turbulent air currents and pressure swings, which often occur when opening hinged doors, are avoided with this application.
- High speed doors reduce the incursion of air and the potentially resulting cleanroom air contamination to a minimum.
The following requirements are placed to achieve a GMP-compliant motorized or mechanical drive design:
- The sliding door leaves are only attached at the upper side on a rolling carriage. Thresholds and floor rails are not recommended.
- The complete drive unit and support rail should be enclosed according to dust protection standards, be sealed as well as possible and be mounted on the side of the wall with the lower cleanroom standard.
- Technically, it is possible to connect the housing to the exhaust air pipe and to extract the particles generated therein (from abrasion) in a targeted manner (i.e. at "negative pressure" compared to the clean room).
- The housing of the drive parts should be easy to open for inspection and cleaning purposes and also be easily accessible for cleaning.
The following safety aspects should be considered or installed
- Safety specifications according to the European harmonised product standard EN 13241. The level of safety can be improved further by using a presence recognition system.
- Emergency stop switch
- Back-up battery or manual emergency opener
- Regular safety checks according to agency, manufacturer and internal safety specifications
In view of the considerable advantages offered by sliding doors in clean rooms, this alternative should be considered more often in practice.
Do you know the legal requirements to be considered when selecting suitable components for pharmaceutical production? Are you interested in new technologies for planning? And would you like to get an overview of the state of the art for wall and ceiling systems, windows and doors as well as floor systems?
Then read the complete Chapter 3.E "Cleanroom Construction Components"in the GMP Compliance Adviser, the most comprehensive GMP online knowledge portal worldwide.
The Chapter 3.E is also available as a GMP-Series e-book A Pharma Guide to Planning and Constructing Cleanrooms.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








