
Regulatory Compliance — Issue Identification
Excerpt from the GMP Compliance Adviser
5 min. reading time | by Mark Tucker
Published in LOGFILE 39/2020
Issue identification is the most critical part of the process and relies on a team of people with high operational and GMP knowledge. All operational and quality areas should be represented and assessed.
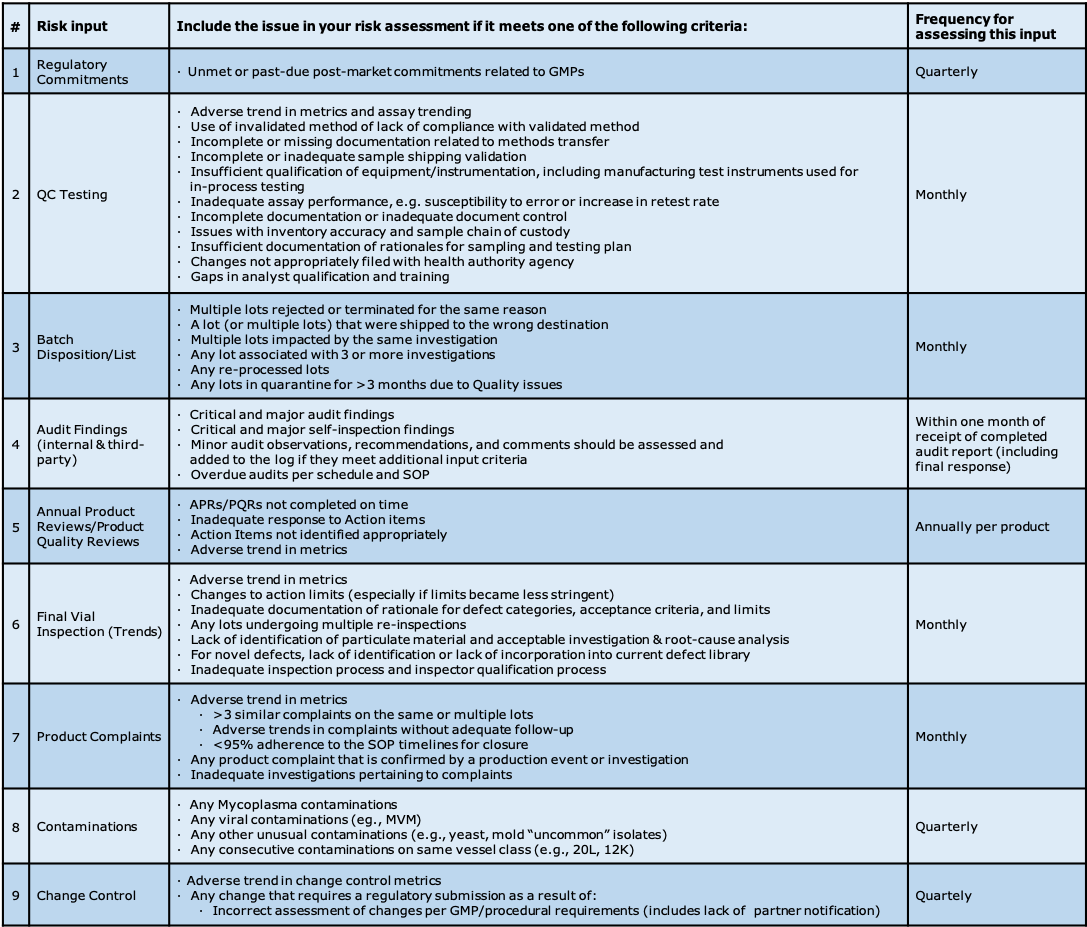
The criteria for what is an issue in each area must be clearly defined, and is dependent on the final purpose of the list (see Figure 1.O-2 ). If this list is destined to be used only for inspection preparedness, you may only want to include issues that would be deemed critical or major by a health authority. If this list is to be used as a quality tool outlining all potential GMP gaps, the criteria will necessarily be much broader. For example, are all discrepancies going to be included on the list, or will only those discrepancies deemed to have a potential product impact be included? In QC, will all Out Of Trend results (OOTs) be included, or only results reported as Out Of Specification (OOS)?
Regardless of how the list will be used, it is imperative that the criteria for inclusion be consistently applied from site to site and from iteration to iteration within a site.
Figure 1.O-2 Some examples of potential risk assessment input values. It is very important to set and understand the boundaries you will use to populate your “risk log”, and at what frequency you expect updates.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







