
Planning, Construction and Commissioning of an Isolator
Excerpt from the GMP Compliance Adviser, Chapter 4.I.6. Isolaters
8 min. reading time | by Richard Denk
Published in LOGFILE 30/2021
Isolator applications and design
Isolators are used in the handling of toxic and highly potent substances and in the production of sterile products.
While the main focus in handling toxic and highly potent substances is on personal protection, in the manufacture of sterile products the main focus is on product protection. Personal protection of operators is also important here if highly active or highly hazardous substances are manufactured.
In contrast to laminar flow systems, where the operator intervenes in the system via an air curtain or, in the case of larger systems, is also inside the laminar flow zone, this is not possible with an isolator system. If manual intervention or operation is required within the system, this is either performed by an internal manipulator or by the operator wearing gloves. Figure 4.I-11 shows an isolator system with glove ports as part of the barrier.

Figure 4.I-11 Isolator system (courtesy of SKAN AG)
The primary purpose of isolators for operator protection is to protect operators from highly active or highly hazardous substances. This type of isolator can be found in nearly all pharmaceutical manufacturing areas. A classic example is in the dispensing of highly active or highly hazardous substances. Dispensing is a fundamental process to be found in all areas of active substance and medicinal product manufacturing as well as bio-pharmaceuticals. In addition to traditional dispensing applications there are numerous further applications such as the packaging of uncoated tablets in blisters (Figure 4.I-12).


Figure 4.I-12 Process isolator for the blister-packaging of tablets (courtesy of SKAN AG)
An isolator for operator protection is constructed as follows:
- Stainless steel housing with connectors for the transport of highly active substances and the disposal of waste
- Glass window with glove ports on the user side to perform operations within the isolator
- Inlet air filter, and in most cases with double filtration of exhaust air
- Isolators for operator protection are operated almost exclusively with negative differential pressure
Planning, design, construction and commissioning of an isolator
To construct an isolator according to user specifications, a 3D model is first designed on a computer. With the 3D model, the first computer-aided simulations of the work steps to be performed can already be carried out and checked. This action includes participation of the customer and manufacturer of the isolator and is an important support for the planned mock-up. Usually the mock-up is built at the equipment manufacturer's site.
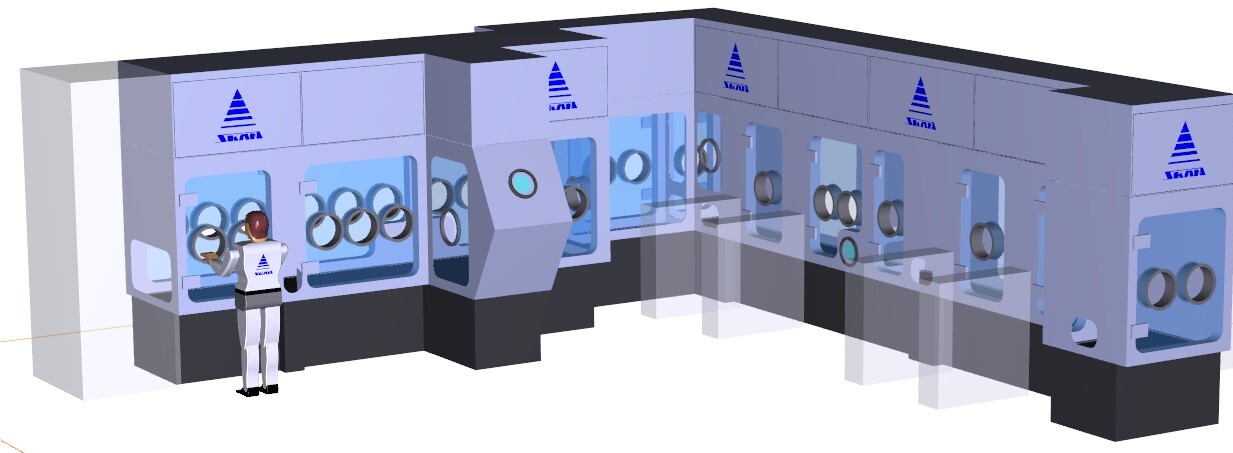
Figure 4.I-13 3D computer model (courtesy of SKAN AG)

Figure 4.I-14 Mock-up model at equipment manufacturer’s site (courtesy of SKAN AG)

Figure 4.I-15 Completed isolator built according to mock-up study results (courtesy of SKAN AG)
During the mock-up process, all manufacturing steps within the isolator are performed by different persons from the manufacturing department, as well as all transfers into and out of the isolator. During the mock-up tests it is important to integrate the future operators and to adjust the execution of the work steps to their requirements. During the mock-up tests, the cleaning and disassembly of parts for cleaning should also be considered, as well as all other work steps that have to be performed with a closed isolator. Before or during a mock-up test, risk assessments should also be carried out in relation to the work to be performed. This concerns the entire process flow, cleaning, GMP compliance as well as aspects of Environmental Health & Safety (EHS).
At the end of a mock-up test, all the positions of the process steps and the transfers within the isolator as well as the glove ports should be fixed. A subsequent modification after finishing construction of the isolator is very costly.


Figure 4.I-16 Performance of mock-up tests (courtesy of SKAN AG)
Once the design of the isolator has been defined, the steel structure goes into production (see Figure 4.I-17). The quality of the steel construction as well as the design of the isolator determine the containment level to be reached and also the cleanability of the isolator and its internals.

Figure 4.I-17 Steel construction framework of an isolator (courtesy of SKAN AG)
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








