
PDA Good Aseptic Manufacturing Conference 2025: The Annex 1 Reality Check
Report from the PDA Good Aseptic Manufacturing Conference 2025, 22-23 May 2025
5 min. reading time | by Sabine Paris, PhD
Published in LOGFILE 13/2025
In today’s feature, our editor Sabine Paris reports from the PDA Good Aseptic Manufacturing Conference 2025 in Basel, where Swissmedic's Paula Walser provided valuable regulatory perspective alongside industry experts. The conference revealed that 25 % of companies remain non-compliant with Annex 1, facing challenges from cleanroom upgrades to contamination control strategies. However, speakers from Takeda, Novartis, and Charles River demonstrated how regulatory pressure is driving innovation – from questioning traditional monitoring methods to advancing digital transformation of sterility testing and rapid microbiological methods. The blend of regulatory insight and industry innovation showcased how Annex 1 compliance is reshaping aseptic manufacturing.
You find much useful information about the topic in the GMP Compliance Adviser, the most comprehensive GMP online knowledge portal worldwide.
The new Annex 1: source of challenges and driver of innovation – an agency perspective
At the PDA Good Aseptic Manufacturing Conference 2025 in Basel, Swissmedic’s Head of GMDP Inspection Operations, Paula Walser, spoke about EU GMP Annex 1, describing it as a challenging yet innovative document.
A PDA survey in 2024 showed that around 25% of pharmaceutical companies are not yet fully compliant with Annex 1.
The key challenges of Annex 1 are particularly associated with older facilities requiring extensive technical measures (longer implementation times):
- Cleanroom classification updates
- Modification of airlocks
- Barrier Technology System implementation
- Procedural and technical improvements
Examples of frequent inspection findings:
- Incomplete Contamination Control Strategy (CCS)
- Insufficient Aseptic Processing Simulation Programs (APS)
- Gaps in material transfers into Grade A areas
- Training/qualification gaps

Annex 1: Driving innovation in aseptic manufacturing:
- Includes new technologies, e.g. single-use materials, automation and rapid methods
- The expected increased use of robotics and fully automated systems will replace many tasks currently performed by humans.
Interesting to know: Swissmedic’s technical interpretation of Annex 1 dated 2023 will disappear soon and will be replaced by a new PIC/S Q&A document reflecting the interpretations of all PIC/S members and also considers industry input
Enablers for aseptic manufacturing
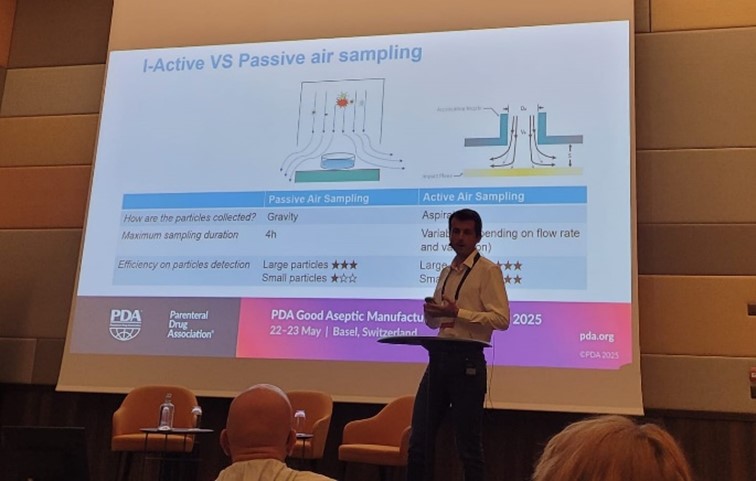
Figure 2 Jules Moussatoff, Takeda, on the PDA Good Aseptic Manufacturing Conference 2025
„Do I really need to use settle plates inside an isolator?“, asked Jules Moussatoff of Takeda during the „Enablers for Aseptic Manufacturing“ session at the 2025 PDA Good Aseptic Manufacturing Conference. His answer was ‘no‘, as the settle plates offer no added value and pose a contamination risk due to the numerous interventions required.
Jules quoted two PDA studies that compared settle plates and continuous microbial active air sampling. Unlike air sampling, the settle plates are not a quantitative method. Settle plates can collect larger particle sizes. Therefore, they are suitable for Grade C or D cleanrooms, but not Grade A.

Figure 3 David Roesti, Novartis, on the PDA Good Aseptic Manufacturing Conference 2025
David Roesti of Novartis spoke about the revision of PDA Technical Report No. 33 Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Methods.
The TR should set out the currently accepted criteria for what constitutes a suitable alternative microbiological method (AMM). Particular focus is given to the validation process (50% of the whole document!).
New or expanded sections:
- Comparability with non-CFU signals
- Changing acceptance criteria
- AI in AMMs

Figure 4 Jon Kallay, Charles River Laboratories, on the PDA Good Aseptic Manufacturing Conference 2025
Jon Kallay from Charles River Laboratories introduced the digital transformation of sterility testing. Sterility testing of complex formulations id challenging, e.g. long-acting depots, ATMPs, implants and nano/microparticles.
Traditional test methods for complex formulations take a minimum of 18-days. Following the digital transformation of the method it was 8-day maximum. An ATP bioluminescence method was introduced.
The advantages of digitising the test method are:
- Provides objective results
- Speeds up incubation
- Removes documentation errors
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








