
Innovations in Pharmaceutical Manufacturing
Conference Report: PDA Annual Meeting, March 15-17, 2021
8 min. reading time | by Thomas Peither
Published in LOGFILE 18/2021
Were you able to follow the PDA Annual Meeting of the Parenteral Drug Association this year? Or were you like many others who spent the whole day with web meetings and have no time or mood for more online conferences?
We attended the virtual PDA Annual Meeting 2021 and in the following I will give you a brief insight into a few presentations that we followed with a focus on GMP.
First of all, I would like to praise the organisation, because it was very helpful to be able to download the presentations before the event so that we could follow them better. It was also easier to formulate questions. The advantage of this approach was evident from the large number of questions and the interesting discussions.
I found the virtual city tour of New Orleans, the coffee breaks with inspiration, music and distraction very inspiring. Other organisers can learn from this!
As PDA President Richard Johnson confirmed, future Annual Meetings will be planned as hybrid events. And I would be happy if, for example, city tours would then also take place locally - but that's just by the by.
President and CEO Richard Johnson, Jette Christensen and Susan Schniepp, conference chairs, opened the virtual PDA Annual Meeting.
Analytical Target Profile and Target Measurement Uncertainty
“ICH Q14 will establish a concept for an overarching analytical method development strategy,” so Robert Mayer from Novartis. Analytical Quality by Design (aQbD) stands for him for a structured method development approach, know-how transfer and a simplified science-based change management.
The analytical target profile (ATP) is a central document to start and support the life-cycle process. It is a prospective description of the desired method performance parameters. And it should be available for all highly critical quality attributes and is a basis for the method selection.
In his presentation, he also looks at the doubts and hindrances in establishing an ATP.
That is the reason that he focused on the TMU (target measurement uncertainty). Robert Mayer identified the TMU as a key tool for defining ATP requirements. Additional points in his presentation are method risk analysis, design of experiments (DoE), analytical control strategy, risk and change evaluation.
This was a good example of how innovative methods can push the industry into the future.
Improving Knowledge Transfer to Reduce Risk and Benefit Patients
Quality risk management and knowledge management are highly dependent. But in practice it isn’t integrated in the pharma industry at all. This was the presentation opener of Marty Lipa, Merck, for the talk with the title “Holding on to What You Know: Improving Knowledge Transfer to Reduce Risk and Benefit Patients”.
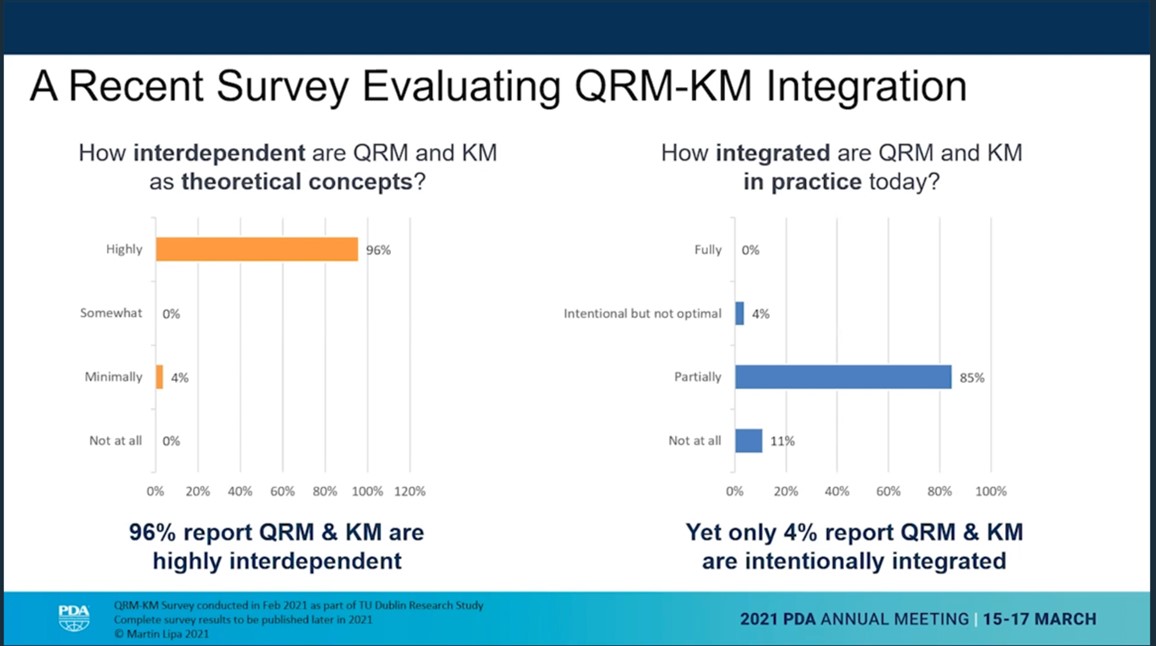
He described the concept of the continuous and perpetual “Risk-Knowledge Infinity Cycle”. Risk is decreasing as knowledge is increasing. Referencing ICH Q10 the risk-knowledge framework could be a solution, if adequately applied.
Marty Lipa described with some very good slides the knowledge transfer challenges, today. The first thing is the focus on minimum requirements to manufacture a batch successfully. But because of heavy documentation orientation, the tacit knowledge gets lost. Additionally, a lot of knowledge leakages in the development and or prior commercial manufacturing experience - a lot of knowledge gets lost.
With that experience, Marty Lipa introduced also an effectiveness toolkit and tested tools to gain tacit knowledge for transfer.
Automated Continued Process Verification
Experience Disruption & GMP live: Continued Process Verification (CPV) is the third stage of FDA process validation. It proves that a manufacturing process operates consistently and compliantly. For each product, regular reports must include statistics for multiple CQAs and CPPs, deviations, trends and explanations. These reports involve a lot of effort.
In his presentation, Ashwin Monian very clearly showed how Merck & Co responds to this effort with an automated solution. The title of his presentation was “Automated CPV Reporting: From Routine Compliance Exercise to State-of-the-Art Process Monitoring”.
Ashwin Monian reports on this groundbreaking project with great enthusiasm. Repetitive and manual tasks were automated and thus made obsolete. New database structures and proprietary software applications were developed. As a result, the basic structure of the corresponding reports can be created and sent for review within minutes.
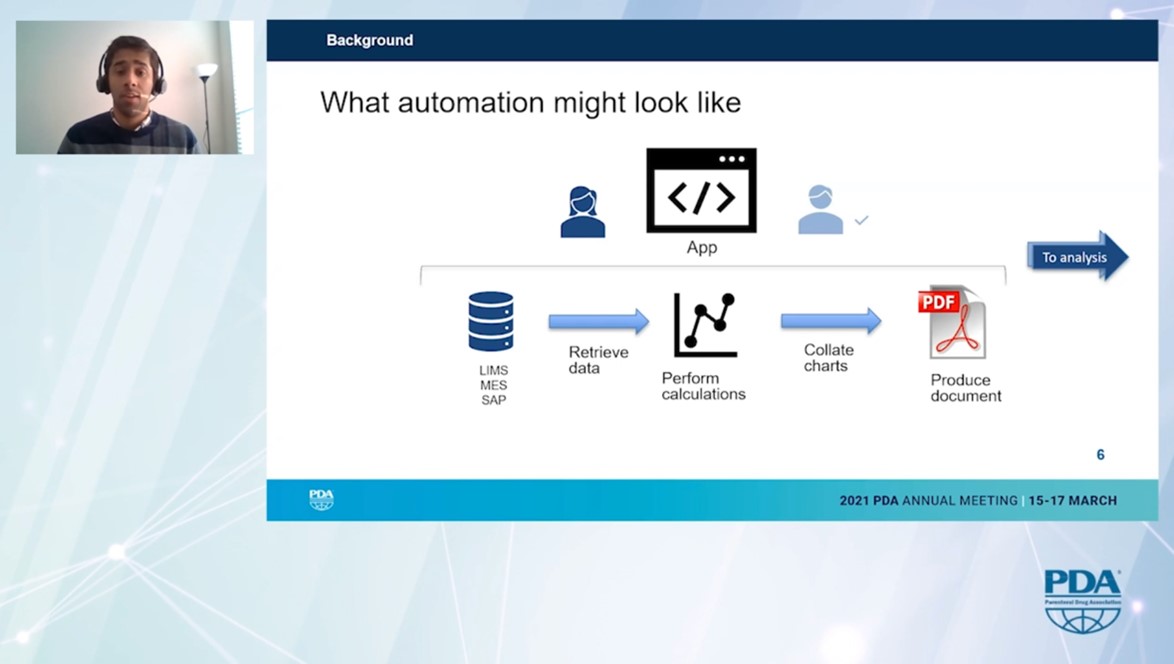
Automation significantly reduces the error rate and massively increases the efficiency of report generation - in the past it took several weeks, today only minutes.
The GMP-compliant, automated CPV software was developed in collaboration with Manufacturing Sciences and IT. It has been implemented at five sites and will be fully integrated across the Group by 2022.
This project is a beacon in the still often conservative pharmaceutical environment. We would like to see more presentations like this, which prove that disruptive approaches in a GMP-regulated environment are possible and worthwhile!
Organisation, Governance and Competency Development
Mauro Giusti, Eli Lilly Italia, gave an impressive presentation entitled: “Organisation, Governance and Competency Development for Successful Parenteral Manufacturing”.
The presentation of the support loops in the organisation was remarkable. He distinguishes between the “variability reduction loop” on the shop floor and team level, the “improvement loop” for the technical team and the “breakthrough loop” on the site level.
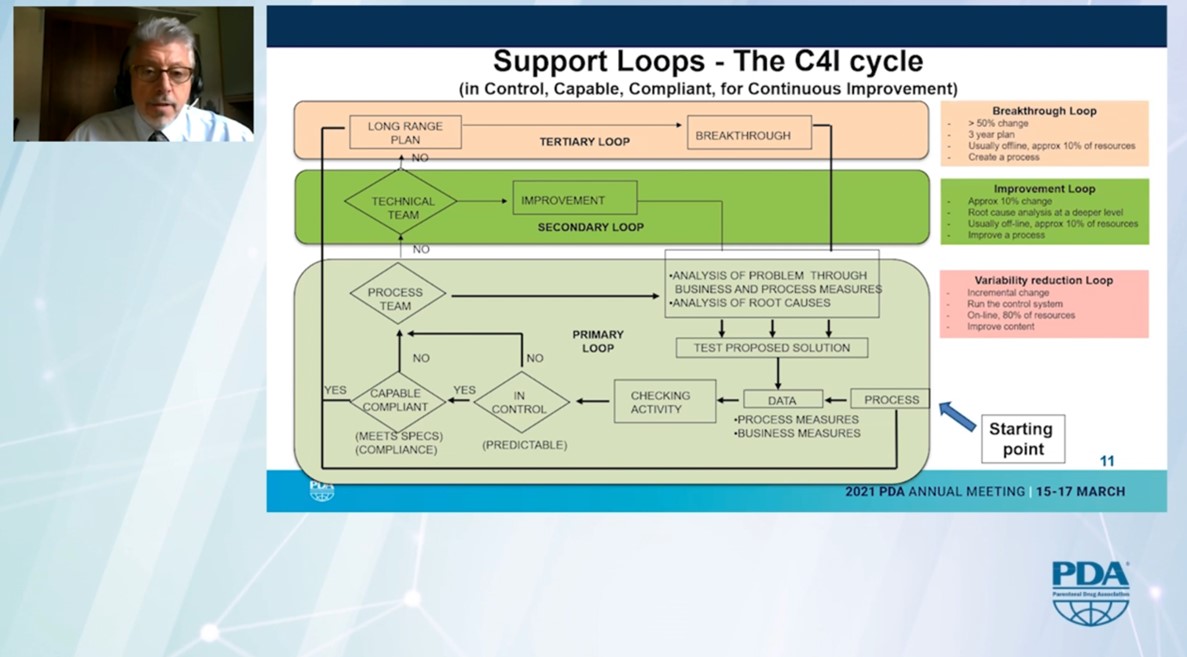
He thus presents a blueprint on how to implement Continuous Improvement or Kaizen in a pharmaceutical manufacturing plant.
Mauro Giusti went much deeper into the organisation of a manufacturing plant for aseptically filled parenteral products. He showed an example of organisation, governance and competence development of employees. He looked at both the hard skills, but also the soft skills and the different career paths that employees can follow.
Thanks to PDA Staff
It was very pleasant and exciting to attend this virtual conference. Many good ideas and concepts offered not only information. Contacts could also be refreshed.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








