
Importance of Supplier Qualification Status
Excerpt form the GMP Compliance Adviser, Chapter 17.D Qualification of suppliers
7 min. reading time | by Christian Gausepohl
Published in LOGFILE 19/2021
Most companies have established different categories (qualification status) for the suppliers of starting materials and packaging materials they use.
The terms are often chosen and interpreted differently. The determination must therefore be made individually for each company. When determining the qualification status, material-specific information must be taken into account in addition to supplier-specific aspects:
- Experience with the manufacturer/supplier
- Experience with the material
- Risk classification for materials and manufacturers/suppliers
- Frequency of goods receipts
The purpose of the different categories is to reduce the internal effort for control and inspection (e.g. analysis required for each batch). Figure 17.D-19 shows the correlation between analysis effort, qualification status and increasing knowledge of and experience with the supplier or material. The risk to the quality of the medicinal product and the processes must also be considered. The reduction in the nature and extent of control and inspection is therefore in keeping with the overall risk assessment.
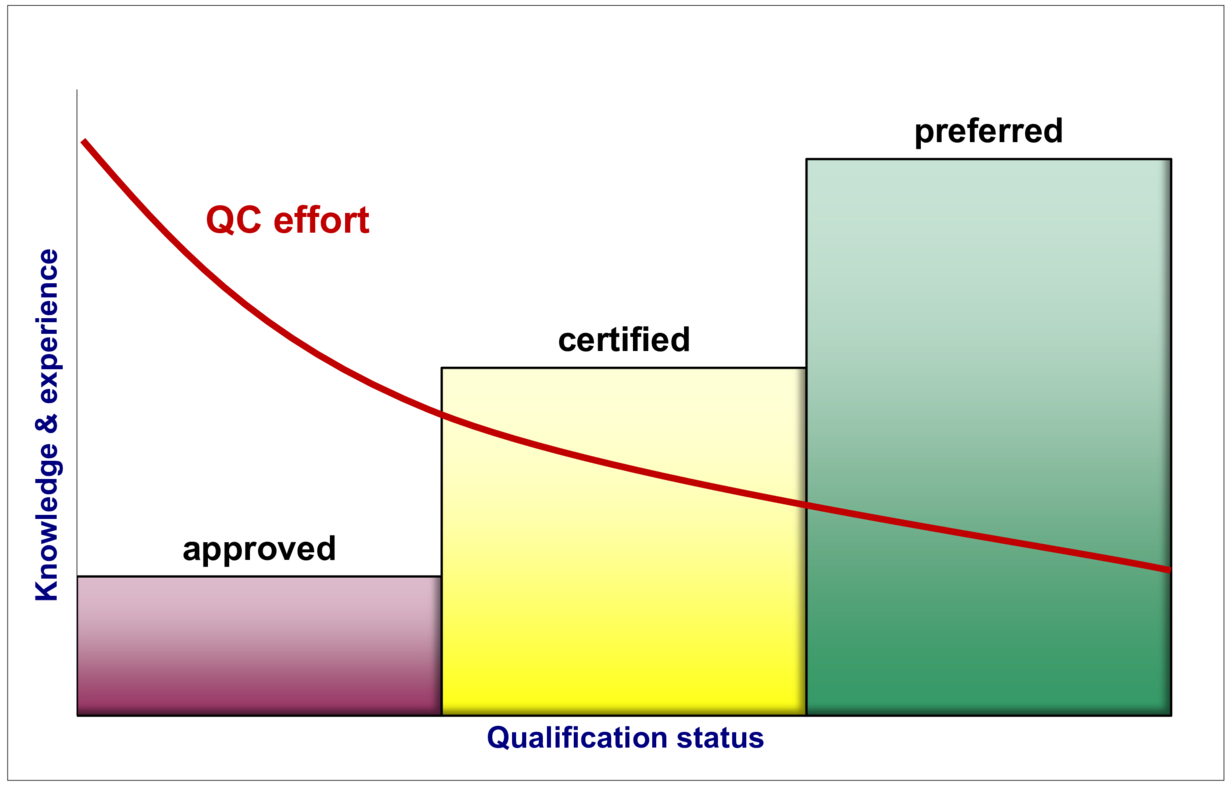
Figure 17.D-19 Example of the relationship between qualification status and QC effort
The following section explains the qualification levels shown in Figure 17.D-19 and their impact on the incoming goods control.
Qualification level “approved”
In a basic qualification (qualification status: approved), the suitability of the supplier is demonstrated in accordance with the defined acceptance criteria.
The testing of samples of 3 batches must yield results in accordance with specifications. For critical materials, successful manufacture of a pilot batch for the medicinal product may be required where appropriate. For each batch of starting materials and packaging materials, it is expected that a manufacturer’s certificate of analysis (CoA) is available.
As part of the incoming goods inspection, a full inspection of the specifications is carried out on mixed samples, i.e. the results obtained by the supplier are not taken from the certificate. An identity test is carried out for each container.
Qualification level “certified”
In the next qualification level (certified), data for a positive batch history is available in addition to the basic qualification. The number of batches required for this and the maximum number of deviations between supplier and internal QC results are defined in advance. A quality audit has been carried out on site over a defined period of time, e.g. 3 years, and shows positive results. The assessment of these results can also be linked to defined acceptance criteria, such as maximum number of audit observations allowed with weighting of criticality (critical, major, minor). This allows the scope of testing to be reduced for selected points in the specifications. A full test is envisaged once a year or for every 10th batch.
Qualification level “preferred”
Building on the qualification status “certified”, the status “preferred” can be achieved at the next level. The prerequisite for this is a lengthy supplier relationship. The reliability of service, the handling of change control activities, proactively where possible, the positive assessment of batch history and the absence of negative observations in inspections by authorities are used for this classification. The amount of analysis required can be reduced to an identity test only. The requirement for full testing once a year or for every 10th batch remains in place.
Figure 17.D-20 summarises the decision-making criteria for classification and their impact on the scope of testing.
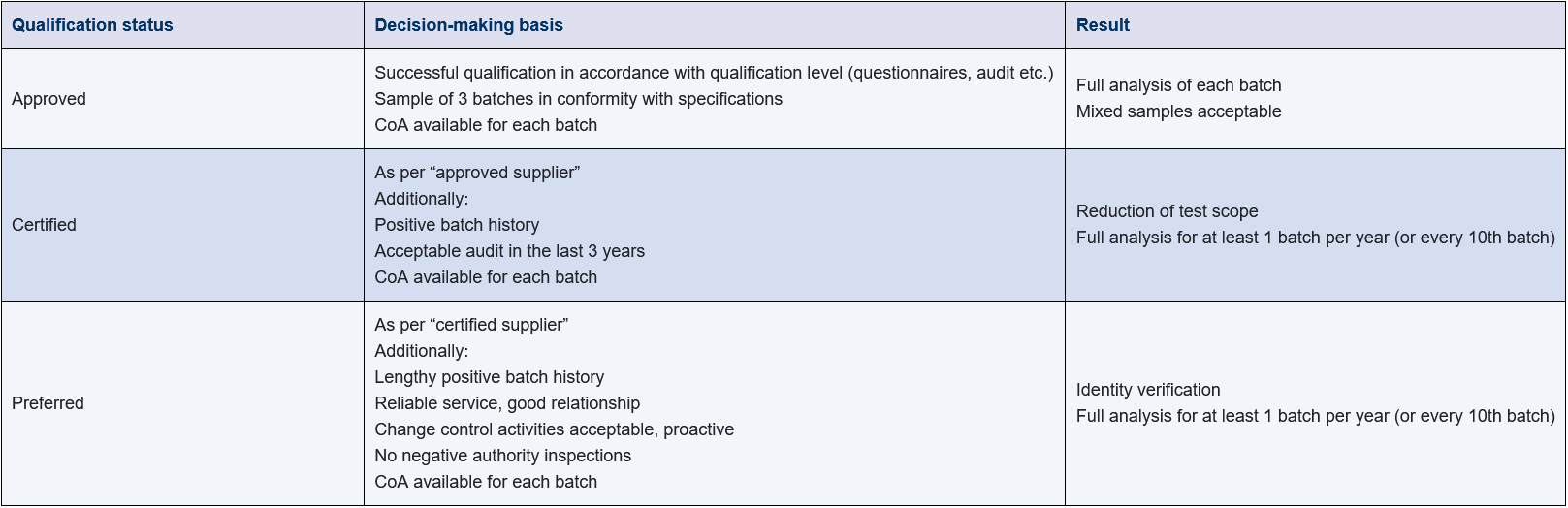
Figure 17.D-20 Example of the relationship between qualification status and quality control activities
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








