
ICH Update Quality Initiatives
A report on the lecture by Roger Nosal, Pfizer, 2020 PDA/FDA Joint Regulatory Conference
7 min. reading time | by
Sabine Paris
Published in LOGFILE 37/2020
For almost 30 years now, the PDA/FDA Joint Regulatory Conference has been taking place once a year. From 14 to 16 September 2020 it was a virtual-only event for the first time. The title of the conference was "The Future Is Now: Effective Quality Management and Robust Manufacturing".
Roger Nosal from Pfizer is the rapporteur of the ICH Internal Quality Discussion Group (IQDG) and presented goals, achievements and guidelines that are currently being updated. The IQDG focuses on technical and scientific aspects in its work. It aims to ensure that the ICH guidelines are up-to-date and reflect the current scientific knowledge.
The ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) has 17 members and 32 observers worldwide and is celebrating its 30th anniversary this year. Experts from industry and authorities work together to eliminate differences in the technical requirements for drug development in the three major pharmaceutical markets EU, Japan and USA. A large number of uniform, recommending guidelines (ICH guidelines) covering all aspects of the quality and safety of medicines, preclinical and clinical requirements have been drawn up and implemented in the participating countries.
Objectives of the ICH
- Reduce the proliferation of the many different regulatory standards
- Harmonised, clear rules based on scientific, robust principles and contemporary standards
- ICH guidelines are intended to ...
- Describe convergence of regulatory expectations - the "what" not the "how"
- Be implemented holistically
- Be accepted by the authorities as definitive and complete
ICH Accomplishments
ICH has greatly improved the global harmonisation of regulatory expectations. ICH has
- Established common vernacular and standard conceptual definitions,
- Introduced and laveraged contemporary scientific justification and risk-based criteria,
- Improved transparency and communication between authorities and industry,
- avoided overly rigid guidelines to allow alternative approaches,
- provided meaningful examples.
Future vision for further harmonisation
- Simultaneous worldwide development and filing
- Mutual recognition or joint review of marketing applications
- Pre-approval inspections follow worldwide standards (e.g. PIC/S)
- Improved post-approval change implementation
- Reduced supply chain complexity
- Reduced drug shortages
- Reduced administrative costs for pharmaceutical industry
- Increased patient access to medicines
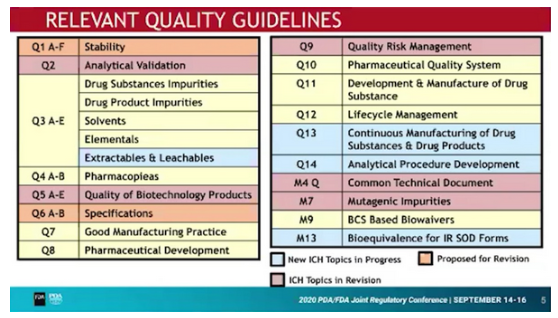
New / to be revised Guidelines
The ICH Internal Quality Discussion Group (IQDG) has identified the following Quality Guidelines which either need to be established or revised:
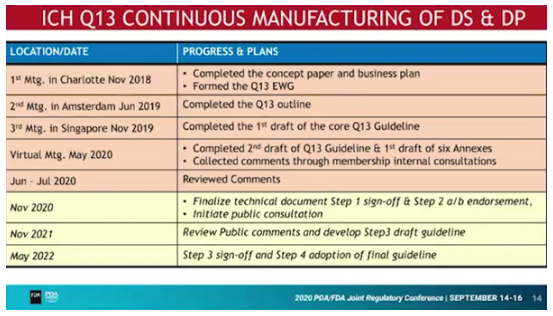
Update on ICH Q13 Continuous Manufacturing of Drug Substances and Drug Products
Objectives of the new ICH Q13 Guideline are:
- Capture key technical and regulatory considerations that promote harmonization, including CGMP elements specific to CM
- Allow manufacturers to employ flexible approaches, i.e., focus on the „what“, not „how“ to develop, implement, or integrate CM for the manufacture of small molecules ans therapeutic proteins
- Provide sensible guidance to industry and regulatory authorities regarding regulatory expectations for the development, implementation and assessment of CM technologies used in the manufacture
It is planned that the document will pass Step 1 (consensus in the drafting ICH Working Group) and Step 2 a/b (consensus in the ICH Assembly) in November 2020, followed by a public consultation. The final guidance could then be published in May 2022.
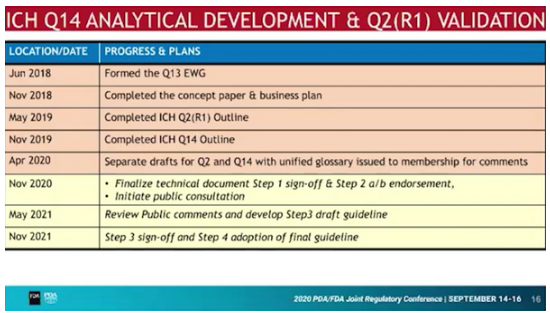
Update on ICH Q14 Analytical Procedure Development und Q2 (R1) Analytical Validation
One topic that ICH has not yet addressed is the development of analytical methods. The new ICH Q14 guideline is intended to close this gap. At the same time, ICH Q2(R1) on the validation of analytical methods will be revised to complement modern analytical methods (e.g. NIR), to accomodate analytical performance criteria, multivariate models and Real Time Release Testing (RTRT).
The timeline is ambitious and final versions are scheduled for November 2021.
The most critical Update: ICH Q9 Quality Risk Management (QRM)
Rogar Nosal attached particular importance to the revision of ICH Q9. Risk management is now routinely applied in all areas of pharmaceutical production. Amendments to the guideline have become necessary as discrepancies have become apparent between how risk management is used in industry and how it is interpreted by the authorities.
What should ICH Q9 pay more attention to?
- High levels of subjectivity in risk assessments and in QRM outputs
- Product availability risks
- Lack of understanding as to what constitutes formatlity in QRM work
- Lack of clarity on risk-based decision-making
Training materials will also be developed to introduce the changes and facilitate their implementation.
The final concept paper, which, according to Roger Nosal, looks very reasonable, is expected to be published in November 2020. The final version of the guideline is planned for 2022.

Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








