
GMP is not ISO 9001 – Where are the Similarities and Differences?
7 min. reading time | by Thomas Peither
Published in LOGFILE 45/2022
Standards such as those in the DIN EN ISO series are not laws, so they differ quite fundamentally from legally binding regulations. Good Manufacturing Practice (GMP), on the other hand, is laid down in laws, regulations and guidelines.
Again and again we receive questions about the comparison of ISO 9001 with GMP, and GMP expert Thomas Peither has done a lot of research, but hardly found anything about it. Today‘s feature presents exclusive information on this important topic.
ISO 9000 & ISO 9001 – voluntary
The ISO 9000 family and thus ISO 9001 describes how the quality of products and services can be improved and the expectations of customers consistently met. ISO 9001 is thus a toolbox for all those who want to or have to establish or maintain quality management in companies or organisations. At the same time, successful implementation can be certified.
It is important to note that there is no legal obligation to obtain certification - even though this is a basic requirement in various sectors if you want to play a role in business life.
GMP requirements – mandatory
Laws, regulations or guidelines, on the other hand, are mandatory for every pharmaceutical manufacturer. The authorities require and monitor compliance with the requirements and can refuse or withdraw the manufacturing authorisation if the specifications are not implemented appropriately.
The national supervising authorities, which are organised differently in almost every country, inspect GMP compliance through regular visits to the factories.
An important difference is therefore that ISO is "voluntary" and GMP is "mandatory".
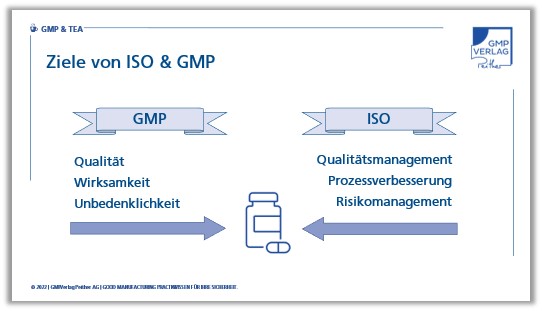
Objectives of ISO 9001 and GMP
The goals of ISO 9001 and GMP also differ: while ISO 9001 focuses on the improvement of products and processes, GMPs focus on the fulfilment of quality requirements.
In principle, ISO approaches solutions via the management and improvement of quality processes with the help of risk management, while GMPs first look at products from the quality control side.
Both sets of rules want to achieve products with high quality, ISO focuses more on the overall company and GMP on the fulfilment of product attributes. A long list that you can certainly continue. How you choose the inspection fields is ultimately up to you. Above all, the selection must fit your quality management system. It can be department-oriented, process-oriented or system-oriented. Each of these variants has advantages and disadvantages and you have to weigh up which one works best in your specific case.
GMPs have changed and developed greatly in recent years or decades. So today you also find the topics of risk management and improvement processes in the GMP world. That was different 25 years ago when I first got involved with GMP. At that time, quality control was much more important than it is today.
Similarities between ISO 9001 and GMP
ISO 9001 and GMP have a lot in common. Whereby in this GMP & TEA episode I am concentrating on the EU GMPs. The complexity would increase enormously if we were to consider US GMPs as well, for example. Simplified, I would say that the EU GMPs have more in common with ISO 9001 than the US GMPs. Commonalities are e.g.:
- Ensuring the production of high-quality products
- Process descriptions that support the goal of high quality
- Introduction of a quality management system
- Involvement of top management
- Focus on production facilities
- Hygiene, monitoring and calibration
- Document management
- Change management
- Supplier audits
- Product quality tests
- Batch documentation
- Material identity tests
- Processes for deviations & CAPA
- Process and product monitoring
- Self-inspections
- Customer complaints
Differences between ISO 9001 and GMP
Here, too, we focus on the EU GMPs and leave out the US GMPs. Differences concern, among other things, method validation and the avoidance of cross-contamination.
The text is a translated excerpt from our German Webcast GMP & TEA.You can find out more about the differences, further details as well as a conclusion and three tips for GMP practice in the complete GMP & TEA webcast episode 25 "GMP ist nicht ISO 9001 (GMP is not ISO 9001)".
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








