
GMP Inspections: Organisation of the Front and Back Office
Excerpt from the GMP Compliance Adviser, Chapter 21.D.4.3
9 min. reading time | by Thomas Peither
Published in LOGFILE 28/2021
The inspection is carried out in the front office and prepared in the back office. The preparation room is used to train and instruct staff.
This interaction guarantees excellent communication and a strong sense of being part of a team. An inspection is a team sport and each team member gives 100% so that goals can be scored in the front office.
Front office
The front office is the inspection room where the inspectors stay during the inspection and are looked after by the staff of the company undergoing inspection. The room is sometimes referred to as the „war room“ – this term was created by the Americans. However, an inspection must not escalate into a "war", so it is better to speak of an "inspection room".
These are the front office roles:
- Responsible inspection lead
- Minute taker
- Subject matter experts (SMEs)
- Runner
Communication with the back office is important for the work carried out in the front office. This is ensured by the runner, but even more so through technical support. Technical support is used to make sure that the team members in the back office know exactly what is happening in the front office.
Back office
The back office is a room that should be directly beside the inspection room. The back office is generally larger with adequate shelf space for documents that are prepared for the inspection. Each document that is moved to the front office is checked in the back office beforehand. The back office is therefore the mainstay of every GMP-inspection. The size of the back office team depends on the complexity of the inspection and the number of inspectors. For simple inspections, sometimes you don't need a back office at all.
The following roles in the back office must be assigned:
- Back office management
- SMEs back office
- Runner
The back office provides answers to the questions that arise in the front office. It is therefore necessary that the experts located in the back office are extremely familiar with quality issues. The work includes:
- Researching documents
- Providing documents
- Examining and checking documents
- Laying out documents in readiness
Important QA, QC and QM documents are normally brought to the back office days before the inspection begins. As a result, they can be quickly retrieved during the inspection. Access to the IT systems of the departments responsible for quality is also provided in the back office. Experienced Inspectors are aware of the support provided by the back office and often visit it after the concluding discussion to thank personnel there for their support.
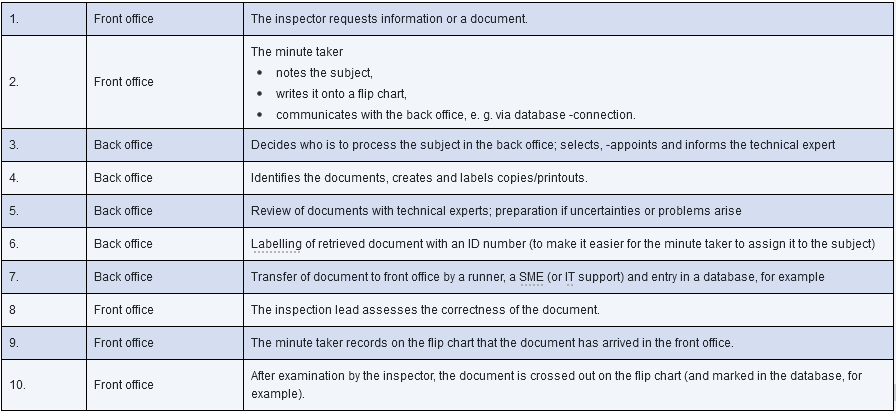
The back office team must prepare itself properly to ensure it can support the front office during the inspection. It should clarify its responsibilities in advance, because this is generally not possible during the hectic day-to-day business of the inspection and dozens of queries from the front office. The situation outlined in Figure 21.D-11 and Figure 21.D-12 is characteristic of an inspection, and personnel in the back office must know who is responsible for which tasks. If the workflow and responsibilities in the back office are properly regulated, this will be reflected in the smooth flow of information during the inspection.
Figure 21.D-11 Interaction between front and back office, © Thomas Peither
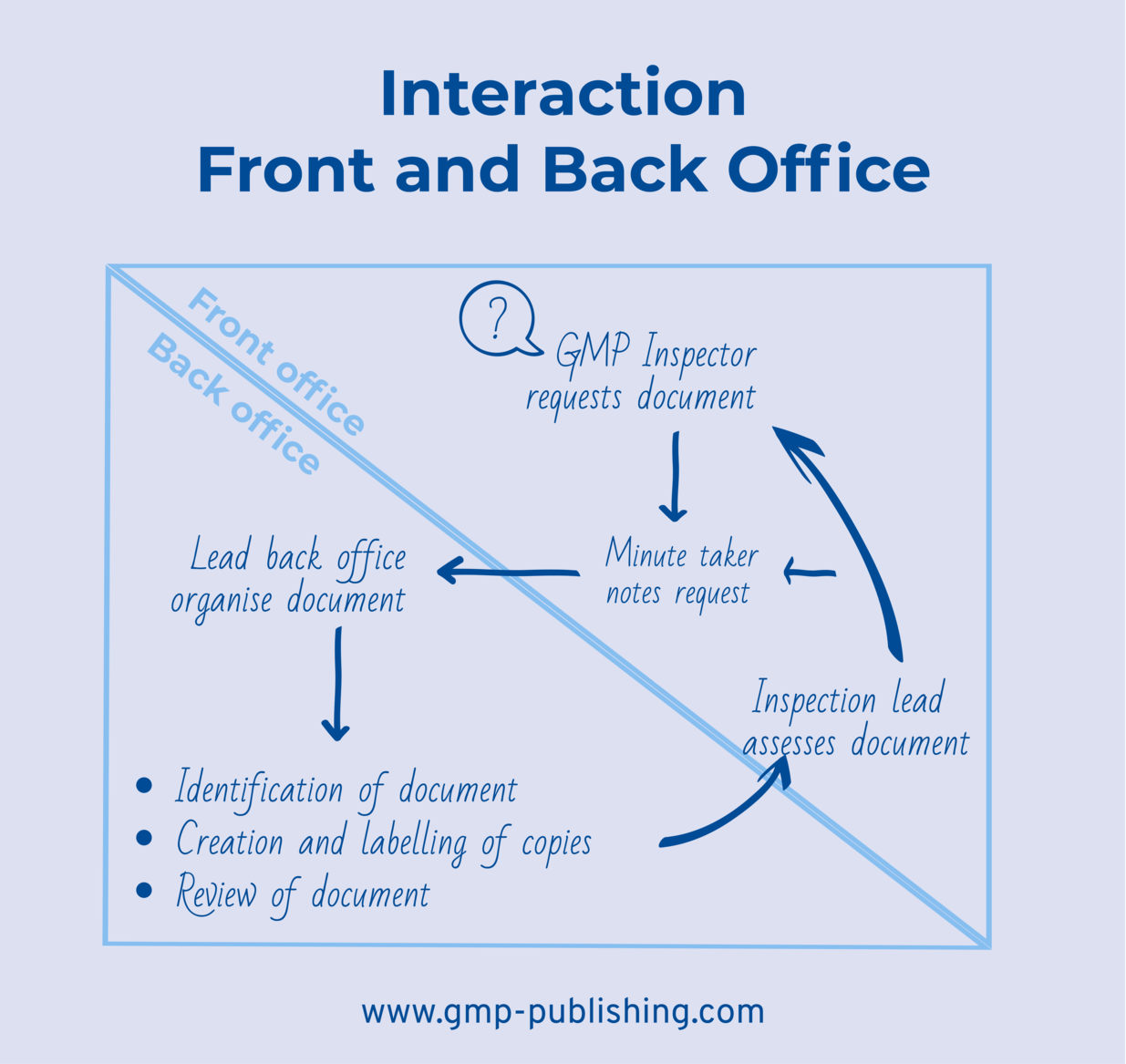
Figure 21.D-12 Example for the interaction between front and back office
Preparation room
The preparation room is a waiting room located between the back office and the front office. Technical experts prepare themselves in this room and can be given some final pointers before their "entrance".
- The preparation room has a number of different functions:
- Technical experts can prepare themselves in the quiet atmosphere (the back office is often extremely hectic).
- Technical experts are prepared for the situation in the front office. How is the atmosphere? Which behaviours have been positively received? What should be avoided?
- The support in the preparation room should be left to team members with the inner quiet required. In this way, the experts can be relieved of their last-minute nerves so they can concentrate on the job at hand.
- Communication platform access should also be available in the preparation room so that the communication between the front and back-office can be followed.
The experiences of the technical experts also are collected in the preparation room after their presentations in the front office. In this way, the preparation room is used as a coaching room to prepare the experts for the atmosphere in the front room.
The preparation room is not mandatory, but it is a good place to escape the partly hectic atmosphere in the back office before entering the front office. Consultants who have prepared the inspection team for the inspection, for example, can have a positive impact on the whole team in this room.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








