
Frequent Deficiencies in GMP Inspections –
Here: Insufficient Maintenance of Rooms
Excerpt form the GMP Series, Frequent Deficiencies in GMP Inspections
9 min. reading time | by Lea Joos
Published in LOGFILE 25/2021
The deficiency
During a tour of the production area, it was discovered at several points that the rooms were not sufficiently maintained:
- Several doors had door gaps of 1-2cm, so that the doors could no longer close tightly and a pressure difference could no longer be maintained.
- In the washrooms, the drain grids were partly fitted with loose screws.
- The capacities, especially for the temporary storage of materials and equipment, were insufficient.
That was the problem
In the present case, the doors were apparently unable to cope with the pressure difference between the rooms. According to the company, comparable gaps in the doors had already been corrected two years ago. The fact that the door gaps now reappeared after the correction was in no way documented, assessed and/or corrected by the company until the time of the inspection.
Neither had the company noticed until now that the drain grids partly had loose screws and that impurities were collecting in the screw recesses.
Having sufficient room capacities available is not classically part of the maintenance measures. However, the aim of maintenance is to maintain or re-establish the functionality. This also includes the "function" of the rooms to be suitable in terms of number and size or to support the logical production process. An inspection of this "function" was not carried out regularly by the company until the time of the inspection. However, during the tour of the production area the following deficiencies were found:
- The production waste was temporarily stored in the corridor.
- Unused equipment was temporarily stored in the aisles.
- Bulk materials were temporarily stored in unused production areas.
- The drying of cleaned equipment took place in the washing room.
- Cleaned small parts were stored in the washroom.
- The weighing room was located on the first floor and the mixing of the different components that followed in the production process took place on the second floor, as the mixing process had been moved to a new, larger room in the meantime. Therefore the raw materials or the intermediate product had to be transported to the mixing room and after the end of the mixing process via several corridors and the elevator.
This bottleneck in room capacity, which has existed for some time, and the changes in the production process due to the commissioning of the larger mixing room had not been evaluated by the company. Up to the time of the inspection, the company had not reacted accordingly.
This is the most common pitfall
Maintenance is a regulatory requirement. However, the concrete implementation varies between different companies and sometimes even within companies between different areas (e.g. laboratory area vs. manufacturing area).
In principle, different types of maintenance can be established. In the case of the above-mentioned deficiency, the company's motto on the subject of maintenance up to the inspection was "If a production employee notices something, it is reported so that it can be rectified". This definition of responsibilities was
- either too imprecise, because every production employee thought "someone else will do it"
- or there was a problem with the implementation of the notifications.
The motto was therefore close to the so-called "run-to-failure" strategy, which is not recommended for the pharmaceutical industry. Why not?
In the case of leaking doors, this means that only when the gap is large enough to be noticed by employees is the problem reported and can be rectified. However, a negative influence on the maintenance of e.g. pressure zones or a possible cross-contamination may already exist before the employees can see the gap with the naked eye. There may be a drug risk.
And even if, for example, the gaps in the door are at some point so large that they could be seen with the naked eye, the focus of the production staff is usually on other things: Setting up the machine, preparing material, keeping the machine running, the time of the next break, the last phone call with the boss, etc.
Next time, go through your home or production/quality control/storage area and be aware of maintenance deficiencies. And then remember how many times you have walked past it without seeing these maintenance deficiencies because you were focused elsewhere.
To avoid this error
If the implementation of reported "failures" falls by the wayside, the difficulties are usually due to the human or financial resources that have to be provided by management and will therefore not be discussed further here.
The necessary test points, which fall under the generic term maintenance, should be recorded in an SOP (depending on the internal regulations, possibly also in different SOPs), so that, for example, sufficient room capacity does not "slip through" during the test.
If the necessary tests and measures are clearly formulated, the question remains as to who will carry them out. This will usually differ depending on the area, the room and the equipment. In order to avoid an unclear delimitation of responsibilities here, the subsequent responsibilities could be clearly defined, e.g. in the course of the qualification of rooms and equipment, as shown in Figure 21.C-9.
Figure 21.C-9 Necessary specifications for the maintenance of rooms and equipment
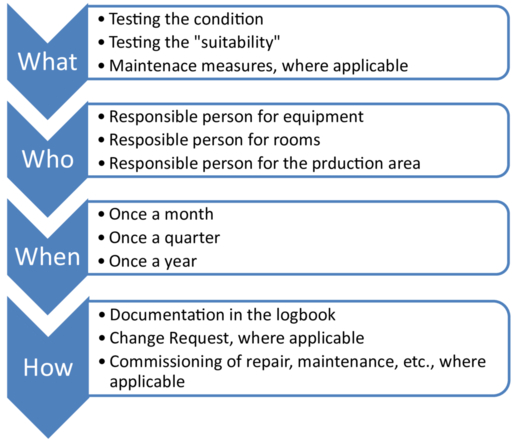
A specification after completion of the qualification could help to ensure that no rooms or equipment are forgotten, as GMP-relevant rooms and equipment must be qualified before commissioning.
In particular, the examination of the "further suitability" of the rooms or equipment in terms of size, number and production sequence should also be carried out regularly (periodic review) and on each change (change management).
The above-mentioned points do not necessarily have to be part of the maintenance. For example, the test of sufficient room capacity could also be an integral part of regular self-inspections, so that changes in the necessary production capacity or in the production process can be reacted to promptly. In any case, it is important to evaluate and document any spatial bottlenecks that may be identified – regardless of the "event" (self-inspection, maintenance, etc.) to which it is linked.
Even if such a spatial bottleneck cannot be eliminated in a timely manner at first, e.g. due to necessary construction measures, at least a risk assessment and a plan of measures should be available.
A separately scheduled and scheduled inspection of the condition of rooms and equipment has the advantage over the "everyone reports anomalies" approach that it cannot be forgotten in everyday production, but must be processed like other (possibly initially prioritised production) orders and then be the focus of attention. By the way: how many maintenance deficiencies did you find in your exercise "Focus Maintenance Inspection" at home or in the company?

Figure 21.C-10 Important specifications for the maintenance of rooms

Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








