
European Safety Referral: Short and Crisp
Excerpt form the GMP Compliance Adviser, Chapter 21.G, Dealing with drug risks and GMP violations
4 min. reading time | by Michael Hiob and Sabine Paris, PhD
Published in LOGFILE 17/2021
A referral is a procedure used to resolve concerns over the safety, efficacy or quality of a medicinal product (or a class of medicines). In a referral, the EMA is requested to conduct a scientific assessment of the products in question on behalf of the European Union (EU).
Safety-related referrals are assessed by the Pharmacovigilance Risk Assessment Committee (PRAC). For centrally authorised medicines a further assessment is carried out by the Committee for Medicinal Products for Human Use (CHMP) or, for nationally authorised medicines, by the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh). All other referrals on human medicines are assessed by the CHMP only.
Referrals can be started by the European Commission, any Member State or by the company that markets the medicine.
At the end of the process, the European Commission issues a decision to all Member States reflecting the measures to take to implement the EMA’s recommendation (see Figure 21.G-1 and Figure 21.G-2).
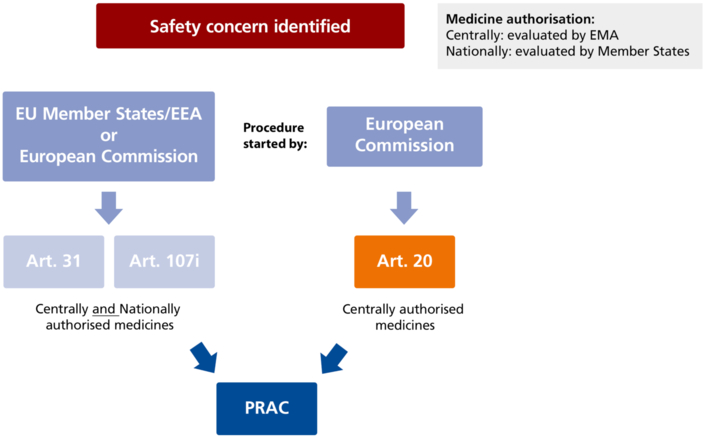
Figure 21.G-1 Start of the safety referral process (Figure created according to EMA presentation on the safety referral process. https://www.ema.europa.eu/en/documents/presentation/presentation-what-european-safety-referral_en.pdf (last accessed on 13. November 2020))
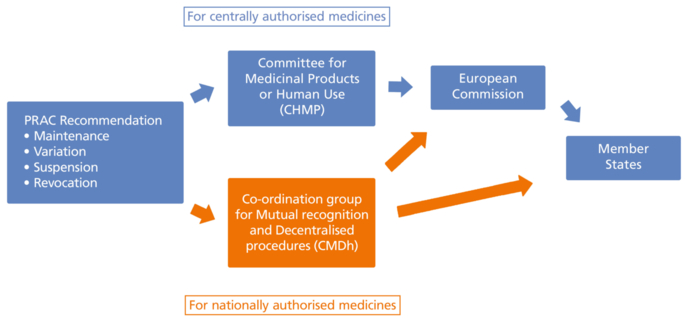
Figure 21.G-2 Safety referral process following the PRAC recommendation (Figure created according to EMA presentation on the safety referral process. https://www.ema.europa.eu/en/documents/presentation/presentation-what-european-safety-referral_en.pdf (last accessed on 13. November 2020))
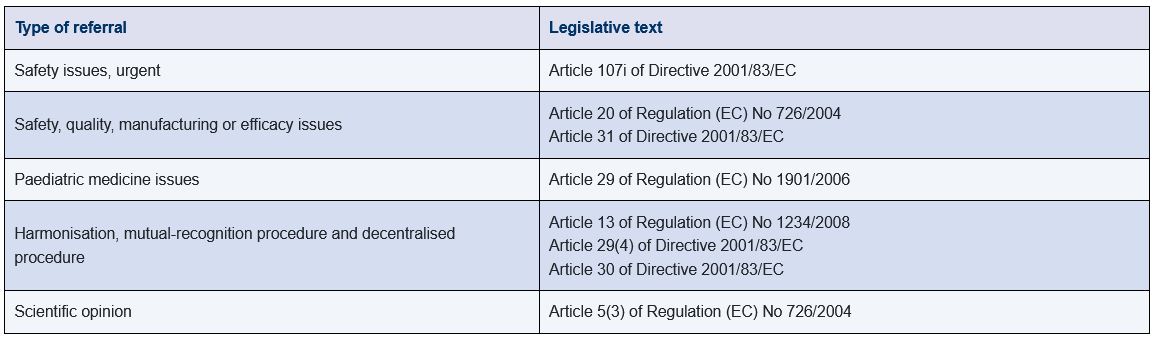
Figure 21.G-3 lists the different types of referrals that are described in the legislative texts that govern how medicines are authorised and monitored in the EU.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








