
Design Qualification for Premises and Air Handling Units
Excerpt form the GMP Compliance Adviser, Chapter 3.J.6 Design qualification (DQ)
7 min. reading time | by Harald Flechl
Published in LOGFILE 22/2021
The design qualification process may vary depending on the project and the client's requirements and may be carried out in two or three design phases.
The evaluation of the individual phases can be concluded with a "phase report". For different tasks, different deadlines for the DQ can therefore result. In the case of extensive projects, it makes sense to summarize the completed DQs of the individual tasks in an overall report.
For so-called fast-track projects the 2-stage alternative with overlapping phases is chosen very often (see Figure 3.J-9) to avoid long interruptions in the planning phase. For example, in a multi-story building it is possible that the DQ for the layout is complete for the lower levels while the detail design of the upper levels is still ongoing. Special attention should be paid to the critical interfaces between the levels.
Figure 3.J-9 Two-phase design

With the 3-phase alternative (see Figure 3.J-10), which is portrayed as the standard method for various project management templates, interim reports are also prepared. DQ is not actually performed until after the detail design stage.
Figure 3.J-10 Three-phase design (FEL – Front-End-Loading – in the area of project management this refers to the practice of establishing a higher level of work at the individual stages with reports for each phase. This serves to reduce the effort associated with late stage changes.)

All documents are to be subjected to DQ which form the basis of the construction work for the manufacturing facility and which are to be reflected in the tender process for the construction of the premises and AHU systems.
During execution of DQ it is to be recorded if the document is
- prepared and available,
- checked versus the URS,
- GMP compliant and
- approved.
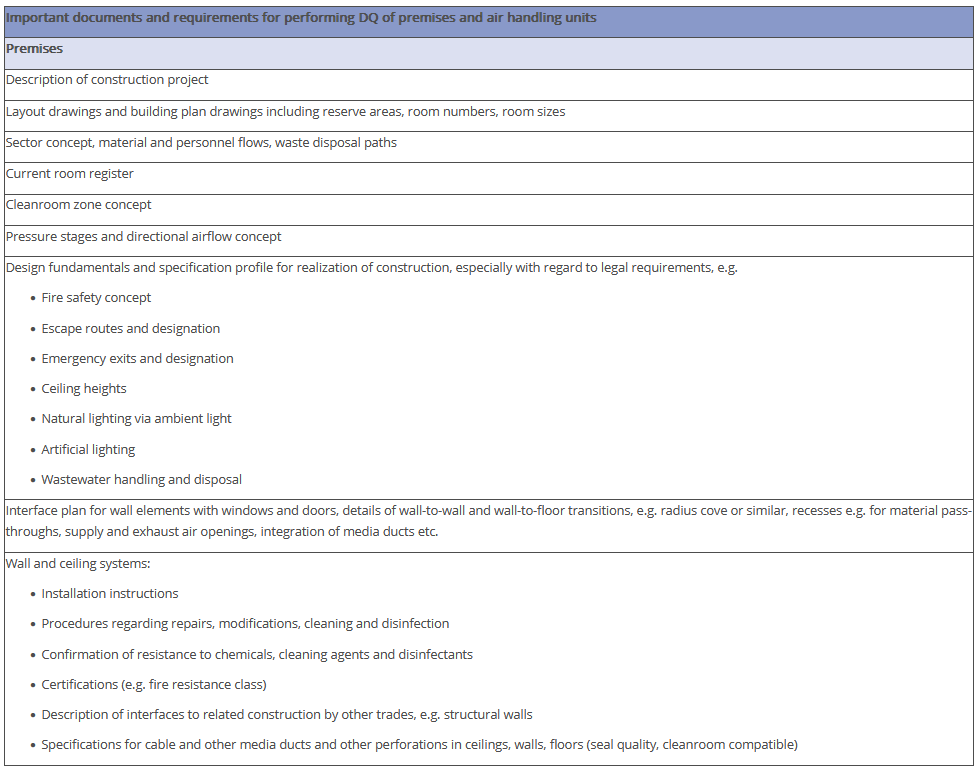
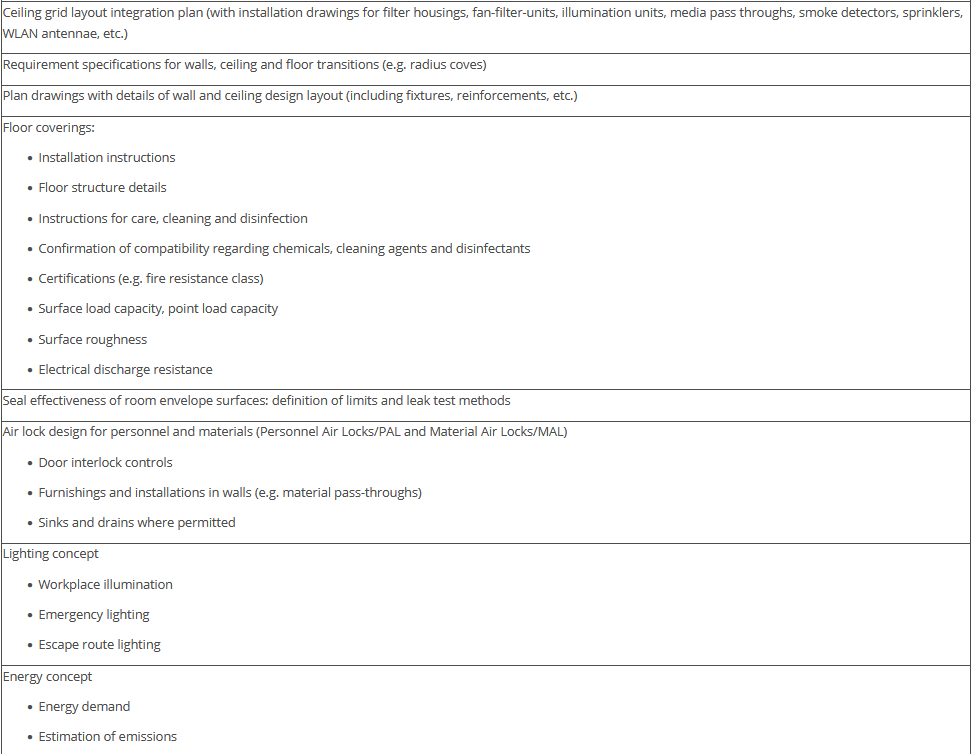
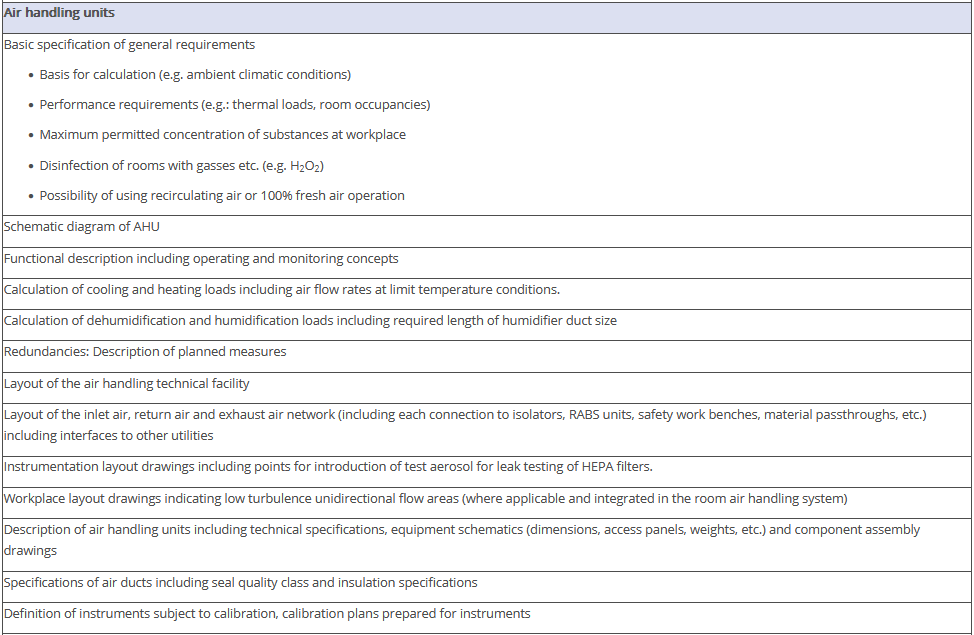
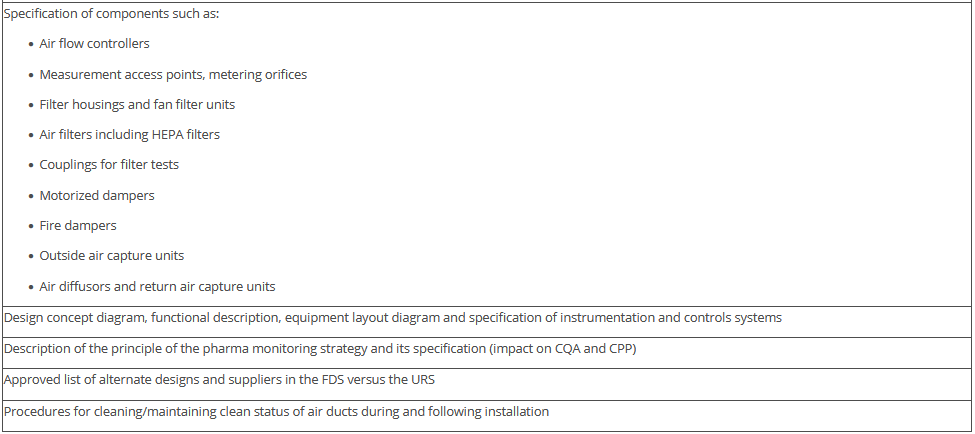
The overview in Figure 3.J-11 does not claim to be comprehensive, nor are all the items listed absolutely necessary. Some of the items shown have no direct connection with the manufacture of pharmaceutical products but deal with general or legal requirements. For example, the roughness of the floor has an influence not only on cleanability, but also on occupational safety (slip resistance), and the fire protection concept is a purely technical safety requirement. The decision as to the extent to which these points are included in the qualification document is the responsibility of the manufacturer.
Figure 3.J-12 Figure 12 IQ checklist for premises and AHU
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








