
Definition of Hygiene Zones
7 min. reading time | by Christine Oechslein, PhD
Published in LOGFILE 27/2022
“While there are clearly defined GMP requirements for particulate and microbial air cleanliness for the sterile manufacturing area, there are no concrete requirements for air cleanliness for the non-sterile manufacturing area. In pharmaceutical practice, terms such as "cleanroom Grade E or F", "grey or black area" and, more recently, the terms "CNC" (classified not controlled) and "NC" (not classified) have become common for non-sterile manufacturing“, explains GMP Senior Expert Christine Oechslein.
Read today's feature to find out how you can define hygiene zones, what requirements apply to D and E areas, and where to find information on the required air cleanliness in non-sterile operations.
The virtual company Peither Pharma GmbH produces exclusively solid forms according to the Site Master File (SMF). No narcotics, CMR substances (carcinogenic, mutagenic or reprotoxic substances), sensitising or highly active substances are processed. The present zone concept, material and personnel flows and all other measures to avoid contamination are adapted to this type of product. This includes the use of the terms "grey" and "white" for the middle or cleanest hygiene zone in the company, which is not standardised or normed. These terms are therefore used in the individual companies for different cleanliness classes and must be defined in the zone concept so that their concrete meaning is clear for internals and externals.
Companies that produce other pharmaceutical forms or process critical substances must adapt their zone concept not only to their own spatial conditions, but also to their own product spectrum!
Three zones are defined at Peither Pharma GmbH:
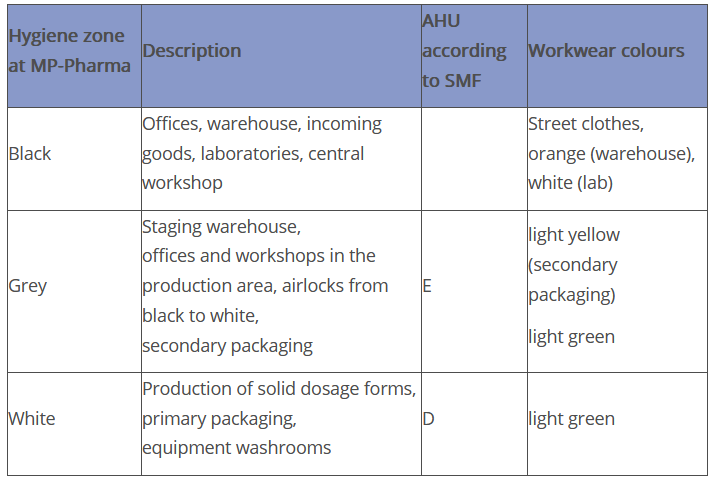
In the SMF of the virtual company Peither Pharma GmbH, "areas of Grade E and D" are defined as follows in the description of the air handling unit under point 4.1.1:
"4.1.1 Brief description of the air handling unit (AHU)
All areas for the open handling of raw materials and/or product are qualified in accordance with cleanroom Grade D. Secondary packaging is carried out in cleanroom Grade E."
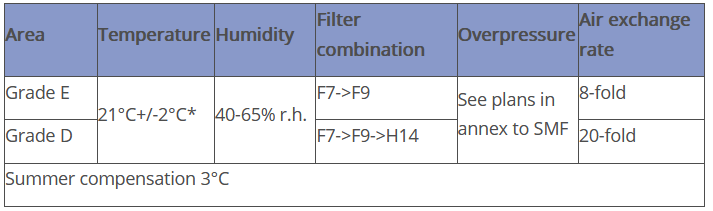
The AHU is designed as a pure fresh air system (two-channel system with variable control of the air volume flows). The air is supplied as turbulent air flow via swirl inlets - the air is extracted via short-circuit-free ceiling and floor extraction points. Details are summarised in the following table:
However, since the last update of the SMF (version 03 of May 2018), the underlying recommendations of the expert bodies have been changed, so that no generally valid criteria for air cleanliness in non-sterile manufacturing areas are currently available.
An internal designation of the hygiene zones with letters (according to PIC/S or WHO) or numbers (according to ISO 14644) is therefore currently not clear and therefore not very helpful for employees.
In order not to confuse production staff when future guideline changes use different letters or numbers for zone designations, the virtual company has decided on a solution that is as simple as possible:
The hygiene zones are only differentiated in black, grey and white; the requirements behind them can then be adapted in the event of changes to the specifications, if necessary. The letters E and D refer to the design of the air handling unit (AHU) as described in the SMF. Since nothing has been changed in the AHU, the information in the SMF remains unchanged.
The air cleanliness requirements for the non-sterile manufacturing area are the subject of lively discussion among experts and authorities. While there are clearly defined requirements for particulate and microbial air cleanliness for the sterile manufacturing area (Annex 1 to the EU GMP Guide and in the Aseptic Guide of the US FDA), there are no concrete requirements for air cleanliness in the GMP regulations for the non-sterile manufacturing area.
In pharmaceutical practice, terms such as "cleanroom Grade E or F", "grey or black area" and, more recently, the terms "CNC" (classified not controlled) and "NC" (not classified) have become common for non-sterile manufacturing.
However, each company must determine for itself which requirements are associated with this.
Helpful indications or recommendations on the required air cleanliness, air exchange rates and monitoring frequencies, warning and action limits could previously be taken from the "Aide Memoire Qualification and Validation in Pharmaceutical Manufacturing and Quality Control" (AiM 07121105) published by the German GMP inspectorates. Unfortunately, these tables are missing in the follow-up document AiM 07121107. There, under 3.1.2, it is only stated in general terms: "Decisive for the quality of the manufactured medicinal products is a sufficient hygiene standard in these rooms, to be defined by the manufacturer. The hygiene standard can be defined, among other things, by limit values for the air and surface bacterial count. Appropriate controls must prove that this standard is permanently maintained (routine monitoring). The position of the measuring points must be determined on the basis of the results of the qualification. The microbiological and, if necessary, particulate air quality in production rooms for the manufacture of non-sterile medicinal products is to be defined within the framework of the control strategy. Following the cleanroom classification defined in Annex 1, the environmental conditions shall be selected in such a way that uncontrolled contamination is avoided. Acceptance criteria and appropriate intervals are to be defined for the operating condition ('in operation') on a risk basis as a component of the control strategy to avoid uncontrolled contamination."
The WHO Guidelines on heating, ventilation and air-conditioning systems for non-sterile pharmaceutical products (Technical Report Series, No. 1010, 2018, Annex 8 and Technical Report Series, No. 1019, 2019, Annex 2) also provide neither proposals for cleanroom grades in the non-sterile area nor concrete information on warning and action limits for germs and/or particles. Obviously, it was not possible to agree on meaningful, worldwide standards, because in draft and previous versions, definitions for cleanroom Grades E and F for non-sterile dosage forms were still proposed and CFU/m³ concentrations for the status "at rest" and "in operation" were recommended. However, both are missing in the current documents.
Furthermore, of little help for the definition of air quality for the production of non-sterile medicinal products is ISO 14644 "Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness by particle concentration", because the ISO classification always refers to all particles - both "living" (germs) and "non-living" (dust, fibres, etc.). However, the ISO standard does not give any values for germ counts (CFU) - but this is exactly what would be required for pharmaceutical classification.
The transition from the black to the grey zone is only possible via personnel or material locks. The grey and white hygiene zones are separated by cleanroom doors.
The text is an excerpt from the German sample SOP 504 Zonenkonzept und Zutrittsberechtigung (zone concept and access authorisation).
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







