
Defect Evaluation Lists and Defect Pattern Libraries Create them yourself – but how?
7 min. reading time | by Felix Tobias Kern, PhD, and Fritz Röder
Published in LOGFILE 37/2021
Defect evaluation lists and defect pattern libraries are popular GMP documents that have been used for more than 45 years. They serve as a rationale for the quality assessment of individual batches and set quality standards for the manufacture and testing of pharmaceutical products.
They represent life-cycle documents that live with the process and must be kept up to date in accordance with any defect patterns that occur.
Pharmaceutical manufacturing companies have the option of purchasing standardised commercial defect evaluation lists and defect pattern libraries. However, these have the disadvantage that they are not tailored to the manufacturing process, the product and the actual defect patterns that occur. Thus, the creation of in-house defect evaluation lists and defect pattern libraries is a suitable option. This article explains how.
The creation of your own defect evaluation lists and defect pattern libraries proceeds in the following steps:
- Step 1: Collection and listing of (potential) product defects
- Step 2: Categorisation of (potential) product defects
- Step 3: Creation of the defect pattern library
- Step 4: Creation of the defect evaluation list
In step 1, all defect patterns that have already occurred and all potential defect patterns are collected. Examples of sources of product defects can be development documents or risk analyses for new products and manufacturing processes, but also defect patterns that occurred during development. For established processes and products, experience from manual sorting, from deviations that have occurred, and from feedback from the market (complaints) can be used.
The defect patterns are listed systematically and each defect pattern is assigned a severity rank (patient risk due to the defect pattern) in a numbering system of, for example, 1 - 3.
In step 2, a decision is made on the basis of the individual error pattern based on the severity rank:
- Charge is acceptable.
- Charge is unacceptable.
- Assign Acceptable Quality Level (AQL) according to DIN ISO 2859-1
For visual management, the defect patterns can be classified in a corresponding traffic light scheme: Acceptable defect patterns are placed in a green area, AQL defect patterns in a yellow area and unacceptable defect patterns in a red area.
In step 3, a defect pattern library is created based on the preliminary work of steps 1 and 2. This serves as an aid for the employees on the production lines or in the laboratory area to interpret the risk of defect patterns that have occurred. The defect patterns are systematically numbered, the defect pattern is shown as an illustration, the traffic light colour is displayed and any follow-up measures are described. These follow-up measures can be:
- Destruction of the batch
- 100% controls (preferably automated)
- Opening a deviation
Sources of images of defect patterns can be actually occurred defects during production or development. Alternatively, these can also be reproduced. It is also important to note in the defect pattern library that newly occurring, unknown defect patterns are evaluated via a deviation and that they are to be newly included in the library. The documents are then versioned accordingly.
In step 4, the defect evaluation list is created for the product. This is a checklist that is filled out by the employees in the optical inspection. The inspection result is entered in it. This checklist also lists all numbered defect patterns with the test method and the necessary sample size, as well as the acceptance criteria. The follow-up measures in the event of non-fulfillment of an acceptance criterion can be taken from the defect evaluation list or a SOP for the documents.
Figure 1 shows an example of a section of a defect evaluation list for packaging.
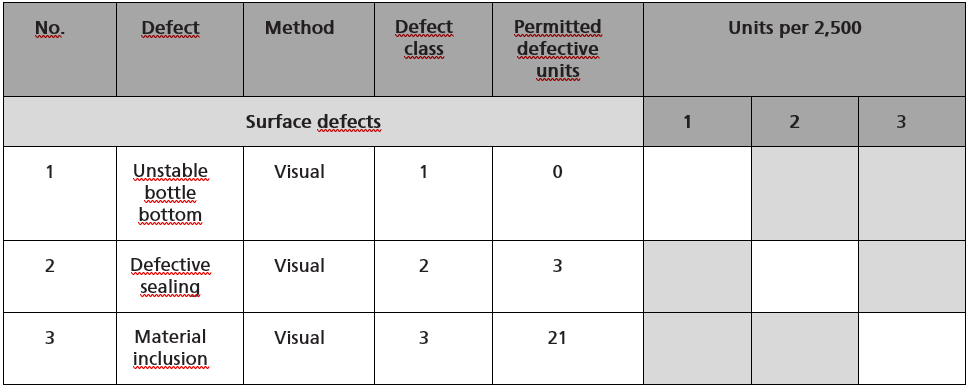
Figure 1
Summary:
Defect pattern libraries and defect evaluation lists play an important role in everyday GMP practice. These documents are used to document and evaluate the quality of a batch. Experience shows: These documents should be created by the company itself following the described steps 1 - 4. In this way, the library and the defect evaluation list can be optimally tailored to the company's own process and product.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








