
Containment in Perfection: The Isolator Technology
Shortened excerpt form the GMP Compliance Adviser, Chapter 4.I Containment (personnel protection) for APIs, solid oral dosage forms and bio‑pharmaceutical products
10 min. reading time | by
Richard Denk
Published in LOGFILE 02/2021
The literal meaning of containment is “holding together” or in its common sense “limiting expansion”. In the active ingredient, pharmaceutical and biopharmaceutical industries it is understood to mean the enclosure of a production process or a dangerous location.
Containment has a dual significance for the protection of operator health and protection of product. The safest containment is achieved with isolator technology.
Where are isolators used?
Isolators are used in the handling of toxic and highly potent substances and in the production of sterile products. While the main focus in handling toxic and highly potent substances is on personal protection, in the manufacture of sterile products the main focus is on product protection. Personal protection of operators is also important here if highly active or highly hazardous substances are manufactured. If manual intervention or operation is required within the system, this is either performed by an internal manipulator or by the operator wearing gloves. Personal protection isolators can be found in nearly all pharmaceutical manufacturing areas. A classic example is in the dispensing of highly active or highly hazardous substances.
Figure 1 shows an isolator system with glove ports as part of the barrier.

Figure 1 Isolator system (courtesy of SKAN AG)
How is an isolator constructed?
An isolator for operator protection is constructed as follows:
- Stainless steel housing with connectors for the transport of highly active substances and the disposal of waste
- Glass window with glove ports on the user side to perform operations within the isolator
- Inlet air filter, and in most cases with double filtration of exhaust air
Isolators for operator protection are operated almost exclusively with negative differential pressure. The essential elements of an isolator and how they work are presented below.
Product transfer systems
There are different transfer systems for the introduction and removal of pharmaceutical products such as active ingredients or utensils, which are connected to the main isolator chamber.

Figure 2 Airlock (courtesy of SKAN AG)
The airlock (see Figure 2) is attached directly to the isolator and is often integrated as part of the main chamber of the isolator. At the front or at the free side wall there is a glass door, which may be equipped with a glove or mittens. There is another door in the junction between the air lock and the main chamber of the isolator. The two doors are interlocked against each other, so that at no time both doors are open. There are different pressures between the air lock and the main isolator chamber. The negative pressure (for personnel protection) in the main chamber is lower than that in the airlock. The airlock is equipped with a supply air filter and possibly an exhaust air filter, or a filter unit between the airlock and the main isolator chamber to circulate the air between the airlock and the main isolator chamber.
To introduce the product or the required utensils, the door at the air lock (to the room) is opened. The negative pressure in the system causes a slight air movement from the outside to the inside of the airlock. The product or utensils are placed in the airlock. After the door has been closed and locked again, work can be carried out in the airlock without the operator coming into direct contact with the substance by wearing gloves. Afterwards the door between the airlock and the main isolator chamber is unlocked and now the door at the main isolator chamber can be opened with the gloves. Using the same gloves or the glove on the airlock, the product or utensils can now be pulled or pushed into the main chamber.
Glass panels and glove port connections
On the operation side of an isolator glass panels are installed with glove ports (see Figure 3). The glass panel is necessary to allow the operator to see what he or she is doing in the isolator. For this reason, the glass panel should be as large as possible in order to be able to see all areas in the isolator. In almost all applications the glass panel can be opened. The removable glass panels make it easier to install or remove large components inside the isolator for the purpose of process or equipment modifications.
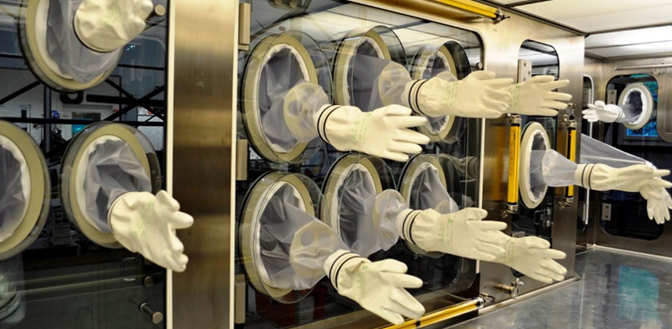
Figure 3 Gloves in an isolator (courtesy of SKAN AG)
To facilitate the opening of the glass panels, a circumferential seal is required between the glass panel and the isolator chamber frame. This seal can be either static seal or inflatable. Both systems are used. From a containment point of view, the seal is an important and at the same time critical component of the isolator.
The same applies to the glove port: If the connection is not properly executed, this can lead to a breach of containment. Gloves on the isolator are sometimes exposed to high stress due to working in the isolator. For this reason, they must be safely and securely connected via the glove connection port. It should also be possible to change the glove without contamination in case of a defect. To make this possible, different manufacturers offer various alternatives.
Glove integrity testing
Depending on the design of the isolator, it may have only a few or numerous glove ports (Figure 3). Each of these gloves has to fulfill different tasks and is also used by different employees. Gloves are subject to wear and tear and should therefore be checked regularly for damage.
Damage to the glove and glove sleeve can occur at different positions. Since gloves are manufactured by a dipping process, the material thickness on the glove is thicker than on the sleeve. Common areas of damage to the glove and sleeve are the fingertips and the spaces between the fingers. The shoulder area on the sleeve is also susceptible to damage.
Use of physical testing makes it possible to validate the testing of the glove and sleeve. Ideally, the glove port is also included in the test to check the correct fitting of the glove and the integrity of the glove on the isolator. The most commonly used method is the pressure drop test. In this method a test device is connected to the glove with a connection port (see Figure 4).


Figure 4 Glove tester (courtesy of SKAN AG)
After the test, the result is automatically reported, and use of the glove can continue or it must be replaced after the test is completed. The test should be performed before and after each production batch or when changing products.
Isolator filter systems
To avoid possible product contamination inside the isolator and to prevent contamination by highly active/highly hazardous substances from inside the isolator to the outside, suitable filters are integrated into the isolator chamber(s).
- With regard to containment, the exhaust air filters from the isolator are of primary interest, since they are most heavily contaminated with the highly active/highly dangerous substance by the suction created in the isolator.
- However, the inlet air filter must also be taken into account, since in the event of a possible pressure drop or before the isolator is cleaned, the isolator may become depressurized and thus the supply air filter also represents a barrier from the inside of the isolator to the outside.
In order to achieve the highest possible retention of highly active or highly hazardous substances, it is recommended to install HEPA (high efficiency particulate air) filters with a very high separation efficiency. It is also recommended to use two exhaust air filters in series as a safety function.
Before use, the filters should be checked for correct installation (integrity). Different methods are available. The special challenge when using filters in containment isolators is how to change the filters without contamination. There are several technologies available to achieve this.
A look into the future: robotic systems
Further protection of the employees can be the use of robotic systems in the isolator. By using robots, manual interventions in the isolator via glove ports are avoided. The use of robots or other automation systems can also reduce the number of gloves or eliminate them completely. The danger of employees working close to the highly active and highly dangerous pharmaceutical substance is thus eliminated. Work and process steps can be monitored from a safe distance. Robots have developed rapidly in recent years. They can now also perform complex operations, and even perform the subsequent cleaning of the isolator and all internals in a validated manner. Figure 5 shows a robot that is used to empty a sterile active ingredient into aluminium cans.

Figure 5 Robot for the manipulation of highly active aseptic substances in aluminum cans (courtesy of SKAN AG)
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








