
Agency Expectations on PDE Determination and PDE Reports
7 min. reading time | by Sabine Paris, PhD
Published in LOGFILE 14/2024
In 2015, with the revision of Chapters 3 and 5 and Annex 15 of the EU GMP Guide and with the publication of the EMA PDE Guideline a paradigm shift occurred in the setting of limits for the validation of cleaning processes. Instead of the previously used criteria, such as 10 ppm or 1/1000 of the therapeutic dose of the foregoing product, the only acceptable criterion is now a toxicological risk assessment based on PDE values.
Whether you prepare the required assessments yourself or have them prepared by external toxicologists, you should know the answers to these two questions: What elements should a PDE report contain? What do GMP inspectors look for?
The following overview shows the specific requirements of national and international regulatory documents for the determination of PDE values and PDE reports.
EMA PDE Guideline
(Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities, EMA/CHMP/ CVMP/ SWP/169430/2012, 2014)
- The identification of a “critical effect” in the establishment of a PDE should be based on a comprehensive literature search including handbook and monographs as well as searches in electronic scientific databases.
- The search strategy and the results of the search must be clearly documented.
- The company should provide a discussion with respect to the critical endpoints of concern and their rationale for the choice of endpoints and dose that is to be used in the derivation of the PDE (No Observed Adverse Effect Level, NOAEL, Lowest Observed Adverse Effect Level,LOAEL).
- The pivotal animal and human studies used for the derivation of the PDE should be sourced to the original reference and reviewed regarding their quality (study design, description of finding, accuracy of the report etc.)
- The PDE determination strategy should provide a clear rationale regarding the adjustment factors that were applied in deriving the PDE.
- In order to provide an overview to the GMP inspectors, the initial page of any prepared PDE determination strategy document should be a summary of the assessment process (see figure 1).
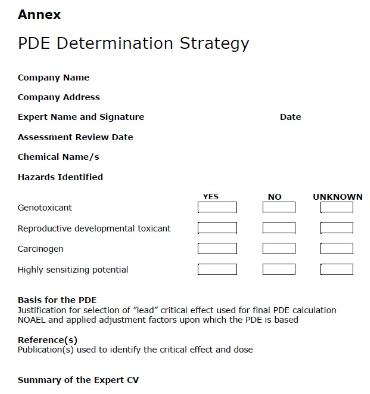
Figure 1 Example for an initial page of a PDE report (Source: EMA PDE Guideline)
Aide Mémoire 07120104 of the German GMP Inspectorates: Supervision of Drug Manufacturers (2019)
Prevention of cross-contamination
Knowledge-based toxicological evaluation of the active substances used:
- Toxicological evaluation according to EMA Guideline
- Calculation of the PDE values
- Initial data basis and validity, scope of research, technical competence, documentation of the procedure
- Identification of critical effects
- Determination of the NOAEL(s) or LOAEL(s)
- Determination of safety factors (also depending on initial values)
- Method of calculating PDE values
- For each application form and critical effect a PDE calculation must be carried out.
- Selection of the lowest PDE value as the basis for calculating the residual limit for the cleaning validation
- Analytical ability to determine residual quantities in the calculated range
- Quality risk management to assess and control the risk and the measures to be defined accordingly
Aide Mémoire 07123001 of the German GMP Inspectorates: Inspection of Cleaning Validation and Verification (2023)
At least the following information should be up-to-date and readily available:
- An overview of the potential degradation products of the active substance and excipients, if applicable, and an evaluation/assessment of the health risk (e.g. literature search)
- For each potentially health relevant residue:
- The underlying health-based exposure limit (HBEL = PDE):
- Separately for different routes of exposure (if next products are manufactured to be administered by different routes)
- Taking into account pharmacological effects, which may lead to an undesirable interaction with the next product as an impurity in the next product
- Including the reference number of the toxicological report
- The underlying health-based exposure limit (HBEL = PDE):
- An overview of next products with a risk of contamination with product contact surfaces including residue limits
- Maximum daily dose
- Route of administration (peroral, parenteral, pulmonary, etc.)
- Minimum batch size in which the next product is manufactured
- In the case of contract manufacturing, the client must give the contractor access to the toxicological and pharmacological data! This is best addressed in the delineation of responsibility contract!
- When determining a "worst case" residue, the toxicological risks of a product and its cleanability must not be considered together!
PIC/S Aide-memoire for Inspection of Health Based Exposure Limit (HBEL) Assessments and Use in Quality Risk Management (PI 052-1, 2020)
An HBEL (PDE) assessment report should have:
- A summary of the decisions, justification and final HBEL figure (HBEL = PDE).
- Be signed and dated by the person(s) conducting the assessment and include or reference their Curriculum Vitae (CV).
- Comprehensive literature search consulted as part of the assessment.
- Clearly documented search strategy Results of the search and commentary on findings.
- Identification of critical effects and points of departure that will be used in HBEL calculations – nonclinical data and clinical experience. Clear rationale for assignment of adjustment factors
The conclusions recorded in the summary should be drawn from specific points made in the text of the document and should not have excluded any points made without clear justification.
Is there a plausible explanation of why the search used was considered most appropriate?
Does the report account for the data found through the search? Are aspects searched, where no information was found related to the product, also recorded e.g. carcinogenicity?
Typically, the lowest HBEL value obtained will be used. If not, this should be justified. If concerned about this, you may wish to seek an expert opinion should the control of the product in shared facilities be high risk.
A rationale for the selection of AFs should be recorded in support of the HBEL calculation. An explanation should be provided for the effect on HBEL for differentroutes of administration (of potentially contaminated products) and for Veterinary Medicinal Products, any susceptibility for specific species.
A CV should provide evidence of the qualifications (typically pharmacy, pharmacology or other relevant pharmaceutical science degree), background in toxicology with reasonable previous experience indetermination of health-based exposure assessments such as:
- Occupational Exposure Limit (OEL)
- Residual solvent
- Elemental impurities (by establishing PDE).
Sources:
Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities, EMA/CHMP/ CVMP/ SWP/169430/2012, 2014 – GMP Compliance Adviser, Chapter C.3.3.7
Aide Mémoire 07120104 der ZLG: Überwachung von Arzneimittelherstellern (Formular 071201_F01_02, optionale Berichtsvorlage, 2019), https://www.zlg.de/index.php?eID=dumpFile&t=f&f=6048&token=3af43aaa1c9ddc1b00e0f7a413505f6e5ae2fc33
Aide Mémoire 07123001 der ZLG: Inspektion der Reinigungsvalidierung und –verifizierung (2023), https://www.zlg.de/index.php?eID=dumpFile&t=f&f=8695&token=a9cb7dd02bee0bf075c73639257e7db467160edb
PIC/S Aide-memoire for Inspection of Health Based Exposure Limit (HBEL) Assessments and Use in Quality Risk Management (PI 052-1, 2020),
https://picscheme.org/users_uploads/news_news_documents/5ef0b5df2ee1a.pdf
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








