
Advancing Data Integrity: New Challenges and Practical Solutions
Report from the PDA Regulatory Conference 2025
5 min. reading time | by Sabine Paris, PhD
Published in LOGFILE 20/2025
What are the challenges and practical solutions for advancing data integrity? Our editor, Sabine Paris, attended the PDA Regulatory Conference 2025 and summarised the recommendations of industry and FDA representatives on the crucial topic of data integrity.
The 34th PDA Regulatory Conference took place in Washington D.C. from 8-10 September 2025 under the motto 'Achieving CGMP Excellence: Sustainable Compliance Across the Lifecycle'.
The sessions highlighted critical CGMP pillars, including quality systems, facility and process design, supplier oversight, industrial modernisation and quality risk management.
I had the opportunity to attend this pioneering conference online, which featured significant participation from the U.S. FDA. For this article, I focused on the crucial topic of 'data integrity', which has evolved from best practice to regulatory expectation.
A data integrity checklist is not a risk assessment
On day two of the PDA Regulatory Conference 2025, Peter E. Baker (President, Life Oak Quality Assurance) made one thing clear: 'A data integrity checklist is not a risk assessment'. Why? Because a checklist treats all workflows as identical.
Data and process governance are no longer optinal – they have become a regulatory expectation (e.g. EU Annex 11, ICH E6 (R3) and PIC/S Guidance). Scientific thinking is based on three elements: hypothesis, experiment and theory. In pharmaceutical manufacturing, the equivalents are design, operation and monitoring. The scientific approach delivers effective governance that is not just a regulatory necessity but a strategic driver of corporate success.
The two enablers for the overall goals of customer satisfaction and profit are continuous improvement, employee engagement and empowerment (see Figure 1).
Peter E. Baker suggested the following practical next steps for data and process governance:
- Map all your workflows
- Create a Validation Master File
- Use one data and process map
- Replace FMEAs with qualitative risk assessment
- Assign clear ownership
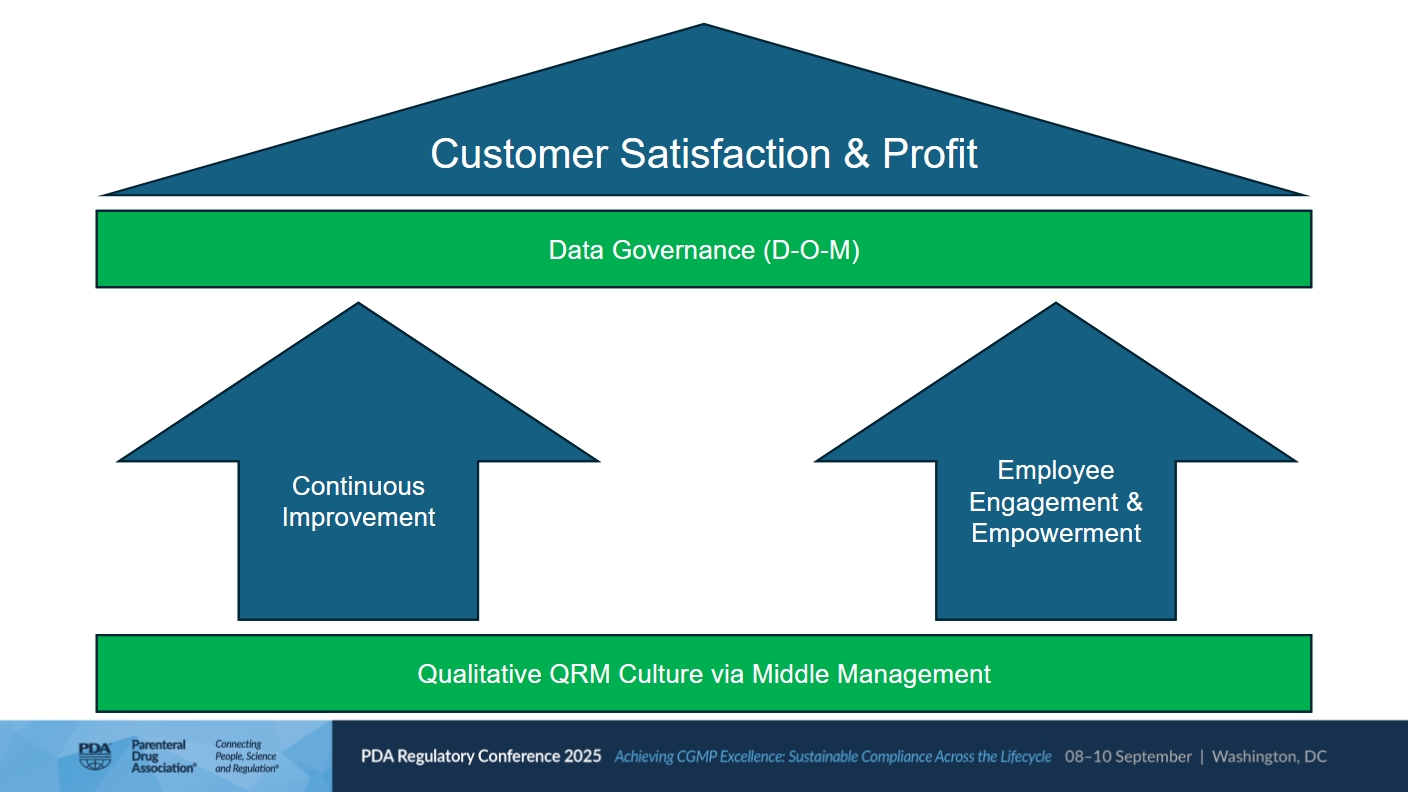
Figure 1 | Enablers of customer satisfaction and profit (Source: Peter E. Baker, PDA Regulatory Conference 2025)
Building resilient data systems
Carmelo Rosa (Director Division Drug Quality, CDER) emphasised that data governance includes data quality, accuracy, consistency, ownership, compliance, and lifecycle-wide management.
He also underlined the importance of resiliency: It’s not about avoiding problems entirely, but about how organisations adapt, recover, and grow from them. A resilient quality culture ensures that failures lead to learning – not repetition.
Since 2014, the FDA continues to find the same data integrity failures. Some firms lack an effective quality structure and reliable systems. The accuracy and integrity of data cannot be adequately ensured. Quality is not a shared responsibility of the entire organisation.
What needs to change?
- Training approaches
- Quality culture mindset with accountability
- Stronger audit programs
- Personnel with expertise in data integrity
- Robust governance structures built on trust, collaboration, and participation
- Data integrity must be seen as a continuous, daily process

Figure 2 | Data Governance (Source: Carmelo Rosa, PDA Regulatory Conference 2025)
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








