
2 Approaches for Implementing ICH Q3D
Excerpt from the GMP Compliance Adviser, Chapter 14.N Elemental impurities
10 min. reading time | by Paulino Alonso
Published in LOGFILE 05/2021
ICH Q3D 'Guideline for Elemental Impurities' applies to finished drug products. It provides the basis for an adequate control of elemental impurities (generally referred as 'heavy metals' or 'metallic impurities'). The publication of this guideline has brought several consequences:
The heavy metals test is no longer included in pharmacopeias.
A scientifically-based risk assessment is required for the evaluation of elemental impurities.
It is possible to perform the implementation of ICH Q3D by two well differentiated approaches:
Component Approach
This approach has advantages from a science and transparency point of view and it is the option encouraged by the EMA (if suitable). In this approach, the contribution of elemental impurities from each component is identified and analyzed. Then, the combined contribution of an element is compared with the PDE in the risk assessment.
In practice, the component approach consists in gathering data from all the components, covering data gaps by analyses of components (or literature, databases, prior knowledge...), and summarizing the concentration of elemental impurities in all the components of a given drug product. Then, evaluation options 1, 2a and 2b are used for the evaluation step and the comparison against the control threshold will provide the results of the risk assessment.
Product Approach
This approach consists in the experimental analysis of batches of the drug product for the presence of any elemental impurities. The results would support the risk assessment to justify a control strategy. Where necessary, the control strategy will include specification(s) for the drug product. Analytical data, without a risk assessment, will not be sufficient to omit a specification for an element. With a risk assessment, depending on its outcome, the number of batches analysed should be commensurate with the risk of the elemental impurities present.
In practice, the product approach requires a risk assessment that determines the elemental impurities to be analysed, the number of batches to be analyzed, and the limits of detection required for each element. With these results, the evaluation is performed according to option 3, and the comparison with the control threshold will provide the results of the risk assessment.
The flowchart shown in Figure 14.N-9 summarizes the different approaches for the implementation.
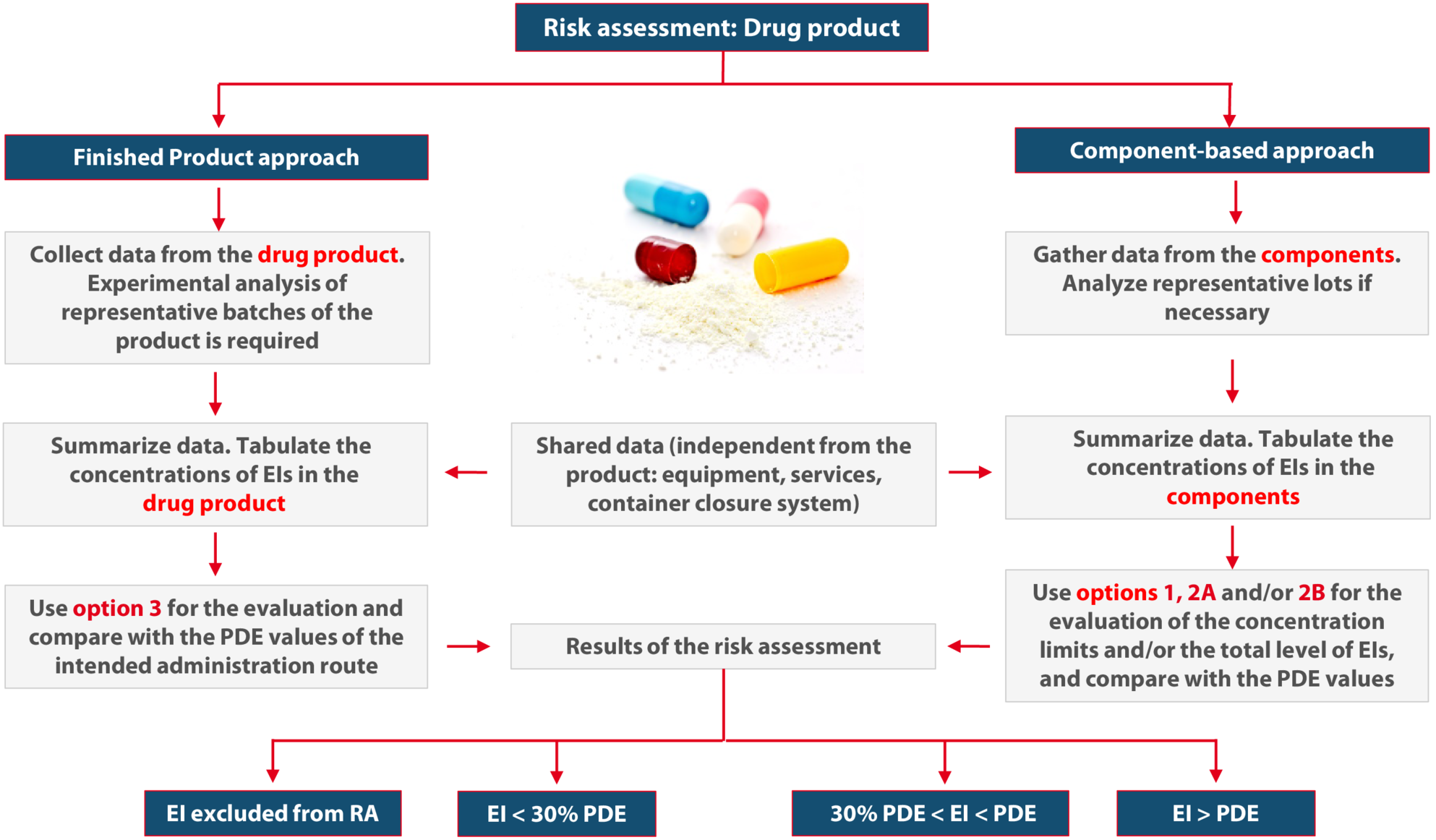
Figure 14.N-9 Summary of the implementation approaches of ICH Q3D
How to choose the appropriate approach
The selection of the approach is a critical decision for the implementation of ICH Q3D. This section discusses the pros and cons of each approach, which may be considered for decision-taking. The facts are summarized in Figure 14.N-10.
| Pros and cons of Component and Product Approaches |
|
Product Approach: Pros:
Cons:
|
|
Component Approach: Pros:
Cons:
|
Figure 14.N-10 Pros and cons of the different approaches for ICH Q3D implementation
Author’s note: From a practical point of view, the component approach is always encouraged. It takes more effort but provides very good results, so it is profitable. However, the drug product approach might be a better option in certain cases:
- if you need 'urgent reports' (it is faster because you don't need to gather the information from all the suppliers);
- if you have 'data gaps' for several components;
- and in certain products in which the risk associated to the manufacturing equipment, utilities, and/or container closure system cannot be ruled out.
Regardless of the selected approach, good planning is the key to achieve a successful implementation of ICH Q3D: potentially the two approaches may be combined (e.g. the risk associated to a given manufacturing line can be addressed through experimental analysis of the 'worst-case' product in that line). In this planning, it is highly recommended to be very systematic in data gathering. It should be good practice that the suppliers are considered as partners.
Documentation management
Irrespective of the approach, there is documentation to be generated in order to comply with the regulatory requirements of ICH Q3D. This section describes briefly how to manage the various documents:
Internal documentation: The complete risk assessment documentation should be kept in-site available for inspection. Authorities may also ask for
- the SOP or equivalent that describes the risk assessment procedure
- the data used and references included
- agreements with suppliers and/or validation data, if applicable
- the change management process and periodic review according to lifecycle management requirements
- the GMP process(es) that make(s) it possible to limit the inclusion of elemental impurities
Regulatory file documentation: The dossier should include a summary of the risk assessment including a brief description of
- approach and strategy used
- identified sources of impurities
- expected levels of impurities that require evaluation
- the conclusion of the risk assessment
- the control strategy, if applicable
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







