
How to Implement Quality Oversight at Shop Floor Level
Excerpt from the GMP Compliance Adviser, Chapter 1.C Quality Assurance Duties
6 min. reading time | by Christian Gausepohl, PhD
Published in LOGFILE 05/2025
The call for Quality Oversight is getting louder – especially in FDA warning letters there is an increasing number of deficiencies observed in the context of quality oversight.
In today’s feature, Christian Gausepohl presents an approach for implementing Quality Oversight at shop floor level as a collaboration between Quality Assurance and Operations. What are the success factors for this model and how could the organisational structure look like?
Read more about Quality Assurance Duties and the implementation of Quality Oversight in your GMP Compliance Adviser, the most comprehensive GMP online knowledge portal worldwide.
Quality oversight at the operational level offers many advantages. The company’s quality culture can be positively influenced through close integration.
But what is the best way to implement Quality Oversight directly at the shop floor level?
If there are already Quality Assurance (QA) functions that are operations-oriented and involved prior to introduction of shop-floor QA and, as a result, they have experience with cooperating at a detailed level, this makes the transition easier for both areas. The joint integration and use of lean management tools in day-to-day operations, such as Visual Management, 5S, Kaizen or Standard Work, makes it possible to grow together, develop and improve continuously.
For the successful introduction of operational quality oversight into the company, there are a number of aspects to consider.
Such an introduction is a conscious decision for cooperation between Quality Assurance and Operations. It offers the opportunity to break down barriers between departments. Basically, it takes time, resources and support from management to make this a lasting success and to realize the potential benefits. The aim is to find a good balance between control functions and cooperation on problems and improvements.

Figure 1: Success factors for operational QA
An essential basis for cooperation is that the QA employees are accepted by the Operations employees. The following factors play an important role here:
- Experience and knowledge of products and processes and the quality requirements
- Communication skills
- Decision-making ability
- Ability to deal with conflict
Important QA tasks are of observational and evaluative nature, meaning that Operations employees may be irritated by negative feedback. This may lead to heated discussions about the competence and experience of QA employees. Soft skills such as the ability to communicate effectively, to make decisions and to deal with conflict are therefore important in addition to professional competence. Careful consideration must be given to deploying QA employees who previously worked in precisely this Operations unit.
The implementation process should be monitored by managers. It must be made clear that the main purpose of monitoring is to improve processes and products and thus ultimately patient safety. This should be communicated as a common goal. Shop-floor QA should not see itself and carry itself purely as a supervisory unit.
Sufficient and consistent staffing of shifts
With the decision to implement shop-floor QA, the necessary resources such as QA personnel and workstations must be provided in the operational environment. The existing working time models, e.g. shift models, must also be taken into account. The required number of QA employees can be calculated based on the specific tasks. It is highly recommended that planned and unplanned absences (sickness rates, vacation days, etc.) are factored in from the outset. Practical execution of support by shop-floor QA can be quite challenging, especially when multiple production lines are running simultaneously and deviations or anomalies are detected.
The consistent staffing of the shifts supports the establishment and development of trust-based cooperation between shop-floor QA and Operations.
It is also advisable to ensure that sufficient and adequate workstations are available for shop-floor QA workers.
Clear distribution of tasks and responsibilities between QA and Operations
In order to arrange the cooperation in a meaningful way and with as little friction as possible, it is necessary to clearly and unambiguously describe the tasks for QA and Operations. Ideally, this is defined for the individual process steps. This also includes the possible decision-making powers in the event of observations or anomalies. For example, arranging the shift plans with prioritization of tasks is carried out by the Operations team leader with the support of shop-floor QA. This concerns the question of the order in which tasks and activities on the production lines are to be executed.
Clear communication and reporting lines
It is advisable to define from the outset how the communication channels between shop-floor QA and Operations should work - i.e. who communicates with whom on which topics and tasks. It should be clearly defined who can request specific support for a process step. Ideally, this should be done via the team leader to enable coordinated priorities.
In addition to the exchange of task-oriented information, it is also important to communicate observations or identified risks. This includes both positive and negative observations, which are passed on directly to employees and team leaders.
In addition to the communication channels, it is important to establish clear managerial reporting lines. Due to the close cooperation, it is expected that Operations and Quality Assurance have a coordinated understanding of the requirements. This can be established in organigrams, taking into account the quality oversight function and active collaboration in Operations. The job descriptions should clearly define the reporting obligations and disciplinary lines for shop-floor QA employees. This is necessary in order to reflect both the independent decision-making authority in terms of quality oversight and the operational involvement of the employee. A simplified example of such an organigram is shown below. Here, the line manager of the shop-floor QA employees is the head of Quality Assurance and there is a further (dotted) reporting line to the team leader of the corresponding shift.
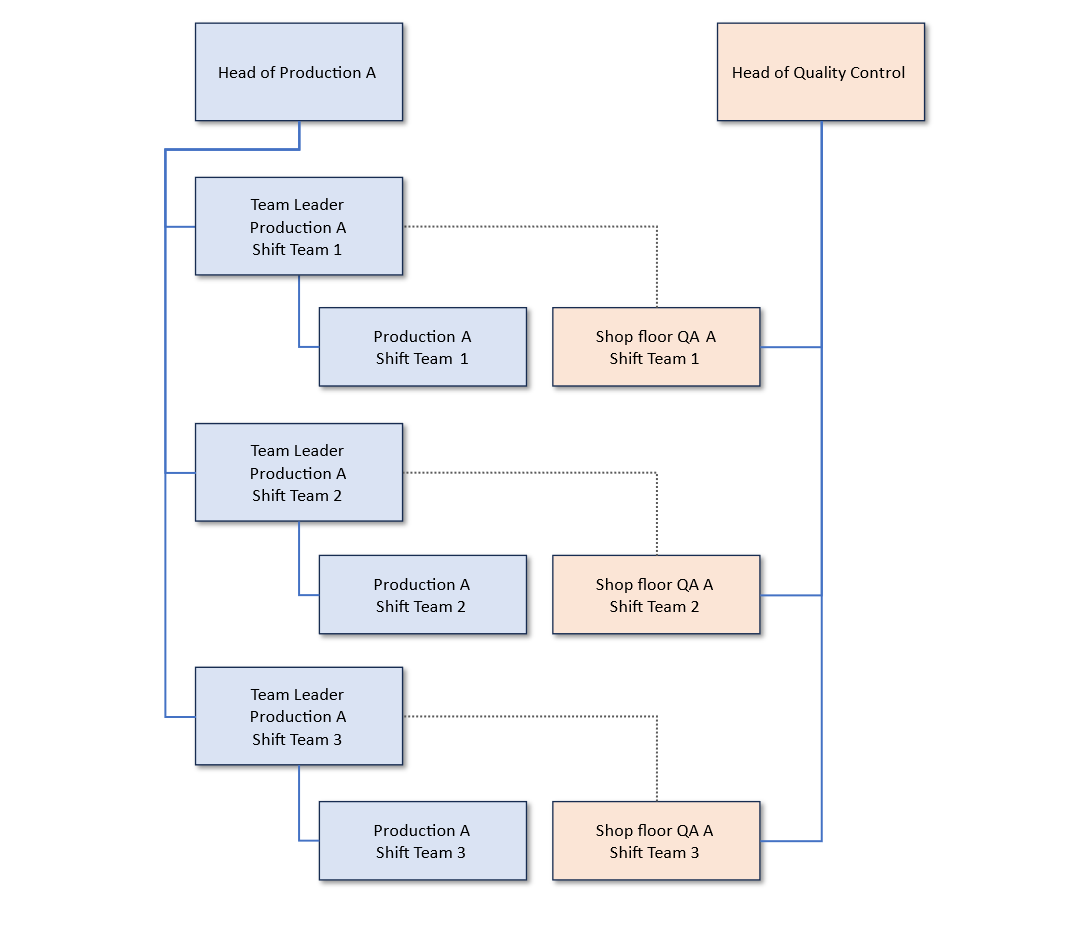
Figure 2: Example of an organigram including shop-floor QA
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








