
Why is it Necessary to “Manage“ Quality?
Excerpt from the e-book "GMP Fundamentals – A Step-by-Step Guide"
6 min. reading time | by Christine Oechslein, PhD
Published in LOGFILE Leitartikel 31/2022
The quality of a medicinal product cannot be left to chance, but must be the result of carefully planned actions. The sequence "planning, acting, evaluating, improving" is referred to as "management".
The four most important principles of GMP are derived from it:
- Every process and every task is described in detail in specification documents (e.g. work instructions, SOPs, checklists).
- Every employee (including managers) carries out the work exactly as defined and reports any deviation immediately.
- The employee records everything in detail while carrying out the tasks so that it is possible at a later stage to identify the who, what, and when of something that happened or was observed.
- At regular intervals, the results are compared and evaluated to determine if there are trends or high occurrences of problems (review, trending). If this is the case, the work processes and specifications must be improved before a quality error occurs (continual improvement process, CIP).
QC/QA – QM/QMS: What is the difference?
The terms quality control (QC), quality assurance (QA), quality management (QM) and quality management system (QMS) are frequently not sufficiently differentiated in daily use. Unfortunately, there are no clear differentiations or definitions in the legislation. Therefore, it is advisable to define these terms within a company and use them consistently. Any person who works with contractors should ask twice when something is not clear to avoid misunderstanding!
Quality Control
In Germany, quality control normally refers to the department that is responsible for carrying out quality testing of raw materials and products. It is also referred to as analysis, quality testing or control laboratory. In the UK and USA, quality control (QC) usually refers to more than just analysis. For this reason, Quality Control departments in American companies often carry out tasks that fall under quality assurance or quality management in Germany, e.g. the approval of specifications or the release of raw materials and products.
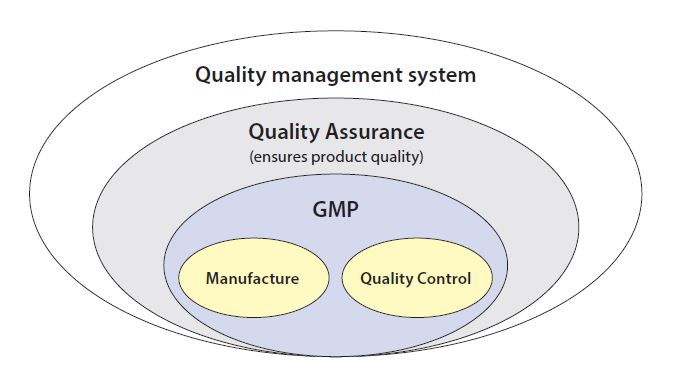
Figure 1: GMP-QM-QA-QC
Quality Assurance
Quality assurance (QA) refers to all measures that are carried out to ensure the quality of a product or service. This involves more than just quality testing of the product. It includes, for example, the approval of SOPs and specifications for the product, deviation management, supplier qualification, process validation, batch record review and the QA measures to be used for the actual product or service. This is described in the QA or QM system.
Quality Management
All planned measures that not only affect the quality of an individual product, but serve to improve processes and services across the board, are referred to as quality management. These measures include quality planning, the continual improvement process (CIP), documentation and self-inspection, and the activities of the executive board (e.g. the definition of quality objectives and management review). In the regulatory requirements, the terms QM and QA are often used synonymously. QM is used more frequently than QA at the present time. The departments responsible for quality assurance and management can be named as quality assurance (QA), quality management (QM), quality unit (QU) or in an other appropriate way.
Quality management system
Everything that is done in a company to ensure the quality of products or services and cross-product quality management must be defined and documented in a quality management system. The terms quality assurance system and pharmaceutical quality system (PQS) are also used.

Why is a quality management system required?
If a pilot notices that something is not right during the final check before takeoff, he or she can prevent something worse happening, but the take-off will definitely be delayed. By the time the mechanics have come on board and found the fault, the take-off slot will be gone.
Sometimes the passengers and luggage are transferred to another flight because the repairs are going to take longer. The passengers miss their connecting flights and the additional expenses (rebookings, hotel reservations, etc.) lead to increased costs for the airline. To avoid additional costs and still guarantee the safety of the passengers, every person involved must carry out their daily tasks properly, and even small problems must be reported and solved immediately so that they can be addressed in time.

It's no different in the pharmaceutical industry. If a manufacturing error is only discovered during quality control of finished product, it is already too late. Injury to patients can still be prevented, but the company has already suffered financial damage. If a medicinal product is affected, the defective product must normally be destroyed (active ingredients and excipients can, under certain circumstances, be reprocessed). It is better to plan and monitor all procedures and work processes from the very beginning in such a way that the product can be expected to meet the necessary specifications.

The aim of a quality management system is to control all of the processes that could affect the quality of a product in such a way that nothing is left to chance: from purchasing the raw materials to shipment of the finished product. Quality control (see chapter 9 Quality Control and market release) is an important part of the quality system, but there are other important elements; for example, the qualification of buildings, facilities, personnel and suppliers, auditing and the proper way in which deviations, errors and changes are dealt with.
The GMP rules stipulate that manufacturers of medicinal products and active ingredients must implement and maintain a functioning quality management system. The GMP rules themselves are not a quality management system, but concrete requirements for the manufacture and control of medicinal products that go a lot further than the usual demands of quality assurance systems.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







