
Challenges Lurking in Manufacturing
A GMP-DIALOGUE of our GMP conference, the GMP-BERATER-Days, in October 2019 revolved around the topic "Challenges lurking in manufacturing". Questions on the topic were asked by participants and answered by the experts in a lively discussion.
8 min. reading time | by Dr. Sabine Paris
Published in LOGFILE 20/2020
Experts: GMP inspector Lea Joos, Christian Gausepohl, PhD, QP and Head of Quality Unit
Protocol: Sabine Paris, PhD
Manufacturing is the origin of Good Manufacturing Practices - here is the core cell of GMP compliance. Many things must be observed and continuously implemented correctly. How do you deal with the challenges of everyday production? Who bears what responsibility? What can be delegated? How do you deal with deviations?
Serialization: How do I deal with when the number of uploaded numbers differs from the number of packages produced?
If only 99 serialization numbers were used in the balancing of a batch of 100 finished packs, this is a critical deviation. A folding carton does not bear a number! In the end, however, this usually does not pose any danger to the patient. The package in question would not be sold in the pharmacy because it cannot be found in the system.

If the balance sheet shows that 101 numbers were uploaded but only 100 packages were produced, the deviation is not critical. A so-called "orphan" number was generated to which no package is assigned.
However, the aim should be to ensure that there are no deviations in number assignment at all. The manufacturer must determine how many deviations (in %) he considers permissible. All deviations can also be assessed as such in a formal procedure. If the number is within the specified range, they do not necessarily have to be formally assessed. However, the generation of a large number of such numbers should be viewed critically in terms of security against counterfeiting.
A root cause analysis is obligatory in case of constantly recurring deviations (e.g. always one number too many). This analysis may not always lead to success, as the questioner reported from her practical experience. In addition, "the serialization software is currently updated almost weekly". This can be critical to quality.
Are there companies that train their employees on dummies?
There are companies that carry out simulated production steps for employee training in a separate environment - e.g. on intervention simulators. The PDA offers special training rooms in the USA. On the one hand, this is considered useful because production does not come to a standstill during this time and possible errors have no effect on products. On the other hand, participants emphasized that training directly in the running production by experienced employees is ultimately the best solution.
For new plants or products, for example, aseptic filling with water is simulated in the actual production environment. One participant reported on film recordings during media fill. As agreed, these will only be viewed if there are any deviations.
What measures should be taken in the event of "employee mistakes"? Are there alternatives to (in these cases mostly used) training?
The measure of all things is an appropriate root cause analysis of errors. The causes can be complex. In addition to actual human errors, device errors and faulty or incomplete specification documents are also possible. The causes of a "human error" (e.g. faulty specification documents, unsuitable processes) should not be overlooked by a hasty assumption of a "human error", but the actual, underlying cause should be determined.
Equipment must be adequately qualified and the related processes validated. SOPs must take into account possible pitfalls. For example, a line that is only fully visible for line clearance above a certain height should be listed in the corresponding SOP for line clearance, together with any necessary measures for smaller employees.
The US FDA sometimes asks in its inspections about the quota for "human error" and the measures taken to reduce this quota.
Employee training should be checked for its effectiveness, especially if the same errors occur again and again. The training courses should be as practical as possible. In the company, an experienced employee can easily assist the person to be trained. The head of production should be in constant contact with the production staff in order to know the actual practice better.
The production staff, especially the head of production, are in a field of tension between the fulfilment of production planning and the observance of quality targets. How can quality awareness be (better) anchored?
The communication of quality objectives can only be achieved through dialogue. In addition, they must be on a par with the production target and not be secondary.
According to criminology, the three decisive factors for non-compliant behaviour of employees could be the following: motivation, opportunity and rationalization. This can be well illustrated in the so-called "Fraud Triangle", which is used in criminology.
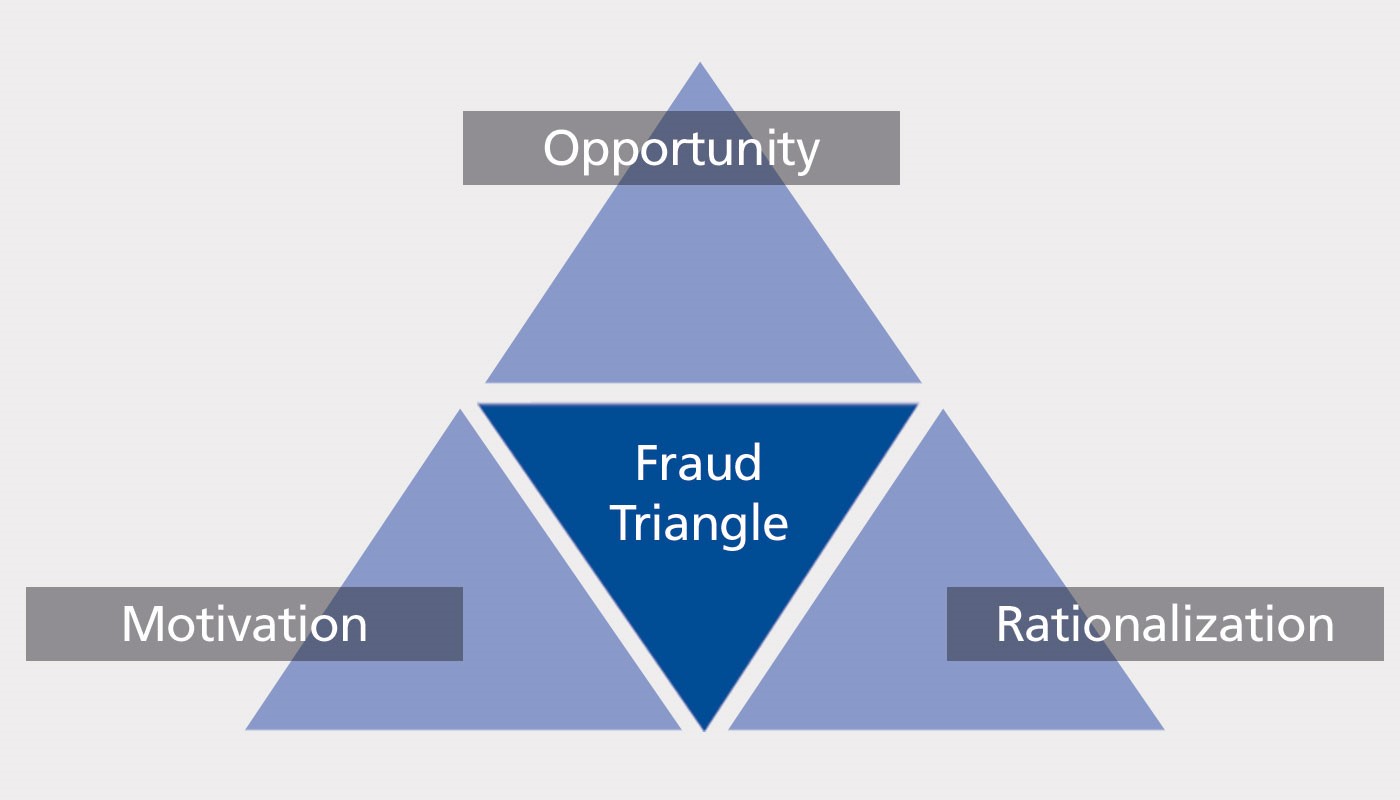
Example for hand disinfection:
- Motivation: "I don't have time to disinfect my hands."
- Opportunity: "Nobody's looking."
- Rationalization: "We are in production backlog. I have to save time. And nothing has ever happened before."
If the employees have a more pronounced quality awareness, this changes the "rationalization". In the best case, the employee omits the rule deviation or in the worst case feels bad about it.
It must be made clear to employees that each individual is responsible for quality. One way to communicate this is through contact with patients or feedback from patients. They report how the medicines produced have helped them. This creates emotional closeness, which is important for quality awareness.
The "opportunity" can also be reduced by increasing the probability of detection. For example, additional measurements or regular observations can be carried out at critical points.
It is important that the quality awareness is exemplified by the management and that realistic (production) goals are set in order to start with the possible motivation ("I don't have time..."). Useful are "Gemba Walks". The management goes to the production site, looks at the processes and talks to the employees. The goals are to gain practical experience, exchange with employees and gain knowledge, as well as to positively perceive the interest in the job and emotionally anchor the importance of the compliance aspect.
Couldn't the supervisory authorities exert more pressure on the management?
The GMP inspectors address the GMP requirements primarily to the functionaries named there (marketing authorisation holder, qualified person, head of production, head of quality control). The management (as a secondary level) has only a few concrete (e.g. management review) and usually higher-level tasks in the regulations, e.g. provision of resources.
Only in the case of overarching aspects/deficiencies will the management be addressed, if necessary, e.g. in the case of an increased number of deficiencies in an area that can be attributed to a lack of resources in this area. However, the authority should not be instrumentalized in this process.
Bottom line:
|
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








