
In-house Logistics: Material Management in Pharmaceutical Companies
Translated excerpt from the 45th Episode of GMP & TEA, a German video series of GMP-Verlag
5 min. reading time | by Thomas Peither
Published in LOGFILE 17/2024
Empty shelves, incorrectly sorted goods and food that has passed its sell-by-date. A supermarket with such problems would have no chance of success. Materials management would have failed across the board. Fortunately, this scenario is rather unlikely. However, it illustrates the importance of efficient and reliable processes to ensure that the right material from the right source arrives at the right place in the right packaging and under the right storage conditions. The same holds true for pharmaceutical operations. Read more about the key requirements for material storage and internal logistics in this week’s brief extract.
In-house logistics encompasses all the processes required to manage and transport materials within a company. This is particularly important in the pharmaceutical industry, where not only efficiency but also compliance with safety and quality standards are crucial. Efficient logistics can help streamline production, cut costs, and ensure legal compliance. The provision of safe and effective medicines relies on pharmaceutical companies prioritising material management and flow. It ensures the availability, quality, and compliance of materials.
This is an extremely complex matter in which several different areas need to be linked together effectively:
- quality assurance and quality control,
- separate storage areas,
- inventory and supplier management,
- technology and system integration and, finally
- production planning.
Storage of materials
When storing starting materials, active ingredients, medicinal products and related health products several basic requirements must be fulfilled:
- separation from other products that could potentially change their quality,
- storage under required conditions,
- in compliance with the FEFO principle and
- avoidance of breakage, contamination, soiling or mix-ups.
Regular stock checks are mandatory, depending on national requirements, at the latest when a batch is used up. Apart from its accounting function, the balancing of raw materials and products has an additional security function. If deviations occur in the inventory that lie outside possible deviations due to e.g. weighing tolerances or underdeliveries, there is a risk of misusage and therefore a production error. In addition, other areas of law, such as narcotics legislation, may also be affected.
This is best done automatically in a computerised material management system, e. g. each time a material movement is posted. For this, it is necessary to determine actual stock levels and define tolerance limits that trigger alarms and initiate necessary investigations.
These limits are typically based on specific, historically determined tolerances. If no material-specific values are available, general limits can also be specified, e.g. 5 % mass tolerance for active ingredients and 10 % for excipients.
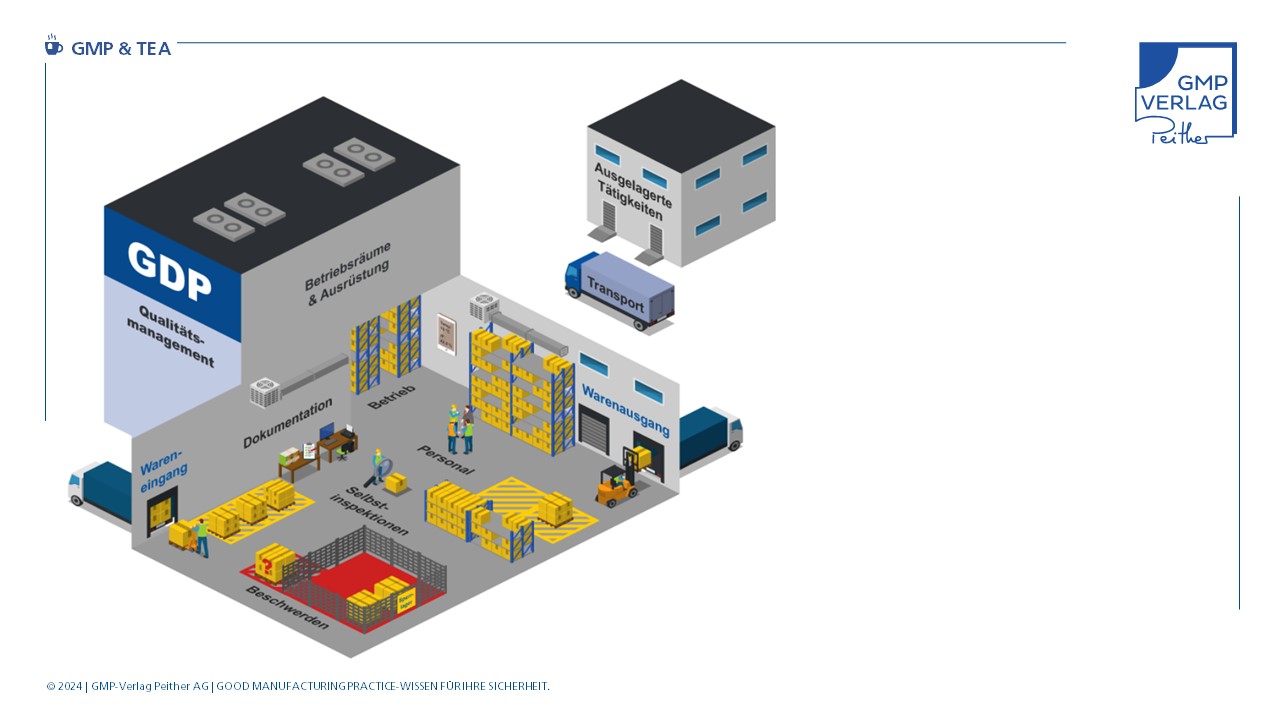
Internal logistics
In the field of storage and transportation, various processes have been outsourced to external service providers in recent years. This is one of the reasons for the introduction of the GDP guidelines. External providers must comply with these guidelines.
Internal logistics, however, cannot be outsourced so easily. This is because most pharmaceutical companies have their own warehouses and staff responsible for proper storage and internal material flow. These employees are ultimately responsible for ensuring that the materials required for production are available at the production line on time. Of course, this requires that production staff have initiated the necessary logistics or transport orders. The storage and transport of materials for production and from production back to a warehouse or from one warehouse to another are subject to GMP requirements and must be documented in the relevant SOPs.
Since the various raw materials often come from different warehouses, their supply must be well coordinated. For ointments, for instance, a large percentage is stored in a 'standard' warehouse, which is not subject to any special requirements. Those warehouses must be in a well-maintained condition and suitable for the purpose, i.e. qualified. Large and well-lit, for example, so that work processes can be carried out correctly and mix-ups are avoided. The suitability of the quality-relevant supply systems installed there, such as air conditioning, ventilation and cooling systems, must also be verified by qualification.
When goods are brought into the production area, contamination must be avoided under any circumstances. For example, wooden pallets, which are often used for closed containers in storage rooms, are not suitable for production areas and must be exchanged with aluminium or plastic pallets beforehand.
On entering the production area, warehouse employees must comply with appropriate changing and hygiene procedures to avoid becoming a source of contamination themselves.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








