
How can the Life Cycle Approach be Applied to Cleaning Validation?
5 min. reading time | by Thomas Peither
Published in LOGFILE 47/2023
The life cycle approach to cleaning validation simply means that control of cleaning effectiveness must be maintained on an ongoing basis.
This type of continuous verification does not end with the revalidation of individual confirmation batches, but requires a control system that continuously provides data on the effectiveness of the cleaning process.
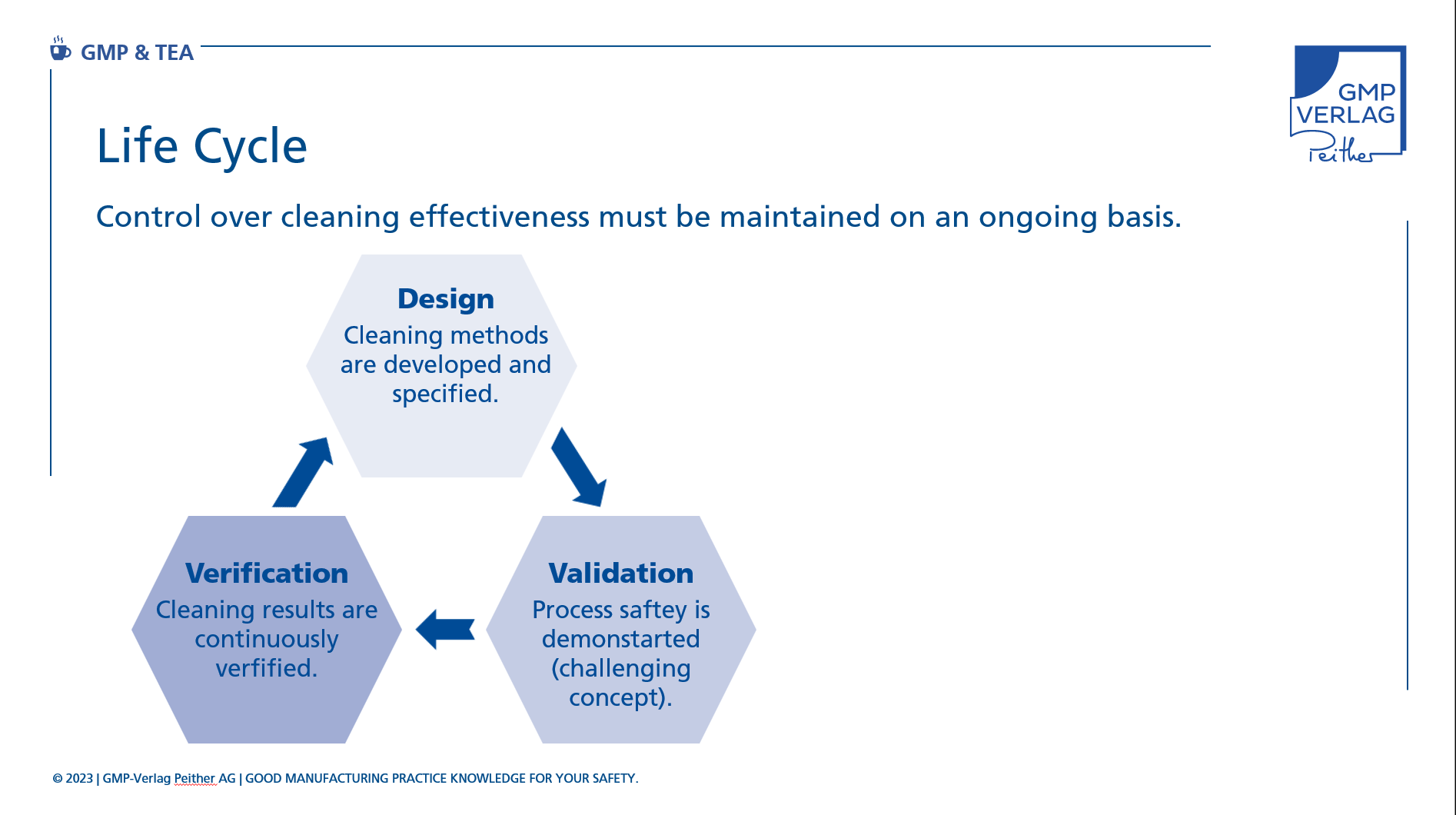
The cleaning validation life cycle is basically divided into three phases:
In the design phase, the cleaning processes for systems or surfaces are developed and defined.
If the "Quality by Design" approach is followed, this will include the definition of a control strategy to control and monitor the cleaning processes in order to ensure reproducible cleaning results that meet specifications.
Classical batch-related cleaning validation is then no longer necessary, as it is continuously and prospectively ensured that the cleaning process leads to the desired result.
In all other cases, where a working control strategy has not been established in advance, the suitability of the cleaning process must be demonstarted in the classical way on a defined number of batches.
In the validation phase, data must then be collected to prove the process reliability of the cleaning.
As part of a so-called challenging concept, this phase can demonstrate that the selected cleaning processes also work under unfavourable but real conditions.
The final phase is the continued verification of the cleaning results mentioned at the beginning.
So much for the theory. What about practice? Which cleaning methods are most suitable? To what extent and at what intervals should each validation tasks be carried out?
These questions lead to the second key point, risk management.

This is the only way to identify and control risks that are not directly related to the cleaning process. These can include the influence of ventilation systems, changes such as component replacement or carry-over via work clothing.
This article is a shortened and translated excerpt from the 38th episode of our German GMP & TEA webcast.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







