
GMP Compliant Plant Qualification: Software Quality Plan
10 min. reading time | by Petra Berlemann
Published in LOGFILE 17/2023
A software quality plan – what is it and how do you create such a document?
In short: A software quality plan describes how a software is to be tested in a planned manner. Typically, the software quality plan is prepared after a user requirements specification has been created and a risk analysis has been carried out for the corresponding software. It checks that the created risk-based user requirements are implemented in the software.
These are the guiding questions when creating a software quality plan:
- What information do I need for this?
- Where do I get the information?
- What does the planning of the test phases look like?
- What is the structure of a software quality plan?
A software quality plan and a software quality report are written by one person who is only involved in writing the plan or report. Another person will carry out the tests. These two functions must be kept separate. A software quality plan does not only assume that the tests can be positive (“software works as desired“). A possible negative outcome (“software does not work as desired“) must also be considered in the plan.
Caution: The software quality plan is not based on assumptions. Instead, it defines acceptance criteria that will determine whether the software passes or fails the test phase.
The following is an example of what a software quality plan might look like:
The software quality plan begins by describing the objective and purpose of the document. The scope of validation is then defined, i.e. the technical system to which the software quality plan applies.
Example: This software quality plan applies to software A123 with interfaces XYZZ1 at site ABC. Ist purpose is to establish the validation status for the software.
It also briefly describes the responsibilities of the validation team and the achievable dates for the different phases.
Example
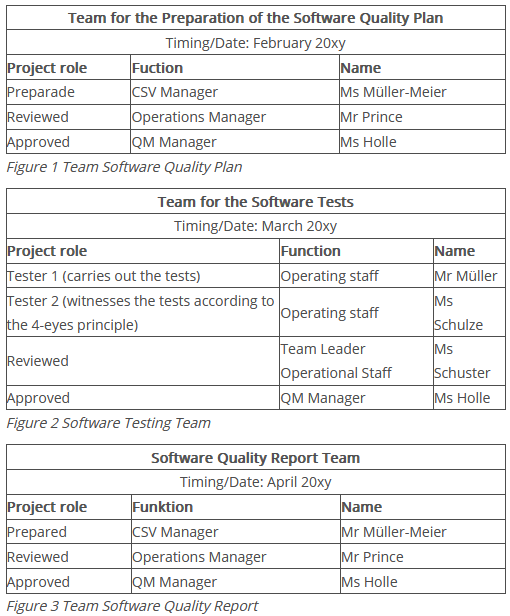
The tabular form is clear and easy to understand.
Training
Persons involved in software quality testing must be trained for the software quality plan. This must be documented by a training certificate.
Software description/computer system description
The software quality plan shall include a brief description of the following aspects:
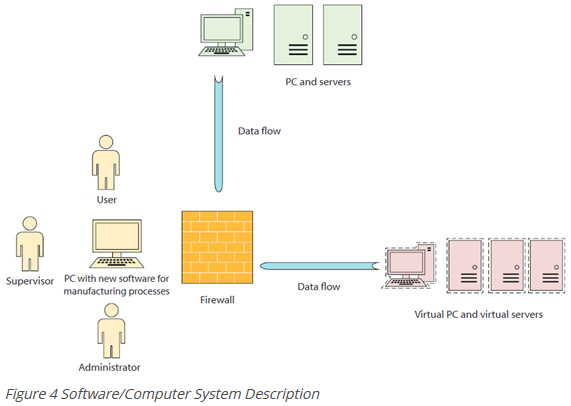
- Which software was procured (name, manufacturer, version)?
- What function does the software fulfil? For example, the control of a manufacturing process or the data management of a database system?
- How is the software or computer system constructed and how is it integrated into existing systems?
This article is an excerpt from the German knowledge portal for equipment and systems qualification GMP:KnowHow Anlagenqualifizierung.








