
Requirements for Balances Used in Pharmaceutical Production
Excerpt from the GMP Compliance Adviser, Chapter 11.B.3.
10 min. reading time | by Christian Gausepohl
Published in LOGFILE 23/2021
When selecting balances, technical aspects such as resolution, precision and weighing range must be taken into account, as must special design features.
The installation location should be protected from draughts, sunlight and vibrations. The correct functioning of the balance must be regularly checked and documented. A distinction is made between a daily check of the weighing range and, for example, a monthly calibration of the entire operating range. In this excerpt from the GMP Compliance Adviser, you will find answers to the following questions:
- What requirements must the installation location of a balance meet?
- Which technical aspects and design features must be considered when selecting a balance?
- How are the calibration limits determined?
Installation location
The installation location of a balance must be designed in such a way that vibration does not affect the system during weighing. Earthing is required to prevent static electricity.
The weighing process should not be affected by draughts. Controlled air flow systems such as laminar flow should be taken into account. Local aspiration can also lead to fluctuation. This applies, in particular, to the low weighing range. To prevent destabilisation caused by the air flow, balances are often placed in booths. However, this increases the cleaning effort because the booth must also be cleaned. The suitability of the overall system is assessed during the qualification of the room or the qualification of the balance. Direct sunlight also can heat up parts of the weighing platform or housing and affect the weighing process.
These factors are particularly important in the case of mobile weighing platforms or small balances because they can be reinstalled in a place that differs from the original, qualified installation location.
Design
To prevent contamination and cross-contamination, the surfaces of the balance including the display and control units must be easy to clean and resistant to cleaning agents. This requires the use of suitable materials and a suitable housing design. These requirements apply, in particular, to parts that may come into contact with the starting materials, e. g. the weighing platform. These parts are usually made of stainless steel, e. g. 1.4571 (AISI 316Ti), cold rolled, electropolished (surface roughness <0.8 µm). Other requirements for the design of balances are summarised in Figure 11.B-13.
Figure 11.B-13 Criteria for selecting weighing platforms

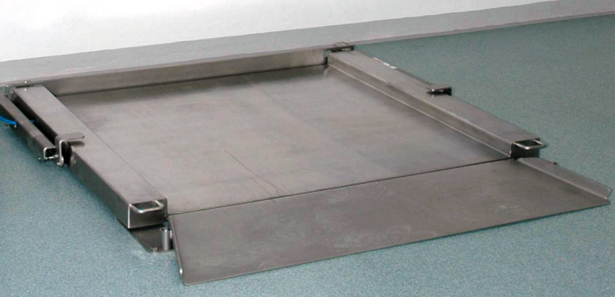
It is important that there are no areas that cannot be accessed, especially in the case of large floor balances (e. g. for containers). Underfloor installations have disadvantages, e. g. poor accessibility and cleanability of the weighing pit. This can only be cleaned properly when the cleaning process is intensified. Folding flat-bed scales where the weighing platform can be unfolded, represent a major improvement because of the hygienic design (Figure 11.B-14 and Figure 11.B-15).
Figure 11.B-14 Folding weighing platform design (image: Sartorius) (part 1)

Figure 11.B-15 Folding weighing platform design (image: Sartorius) (part 2)
Characteristics
Balances and measuring equipment of an appropriate range and precision should be available. Their suitability is confirmed during the qualification process. The aspects that need to be taken into account are discussed below (see Figure 11.B-16).
Figure 11.B-16 Technical aspects to be considered when selecting balances

The individual requirements depend on the intended processes. They are defined at URS level (user requirements specification) or during the design qualification.
Resolution: The resolution describes the smallest difference between two measurement values that can be read on the display. In the case of digital displays, this is the last digit. The resolution should be 5 to 10 times better than actually required.
Accuracy: The accuracy describes the relationship between the displayed weight and the actual weight of the load. It must be adjusted using internal or external weights and checked (calibrated) using suitable external weights at the place of operation on a regular basis.
Linearity: Linearity describes the accuracy across the weighing range. This is, in theory, a linear correlation. Deviations are called linearity errors.
Precision: Precision is the deviation of individual results when repeating a number of consecutive weighing processes. It is generally expressed as a relative standard deviation. Precision is an important parameter for evaluating the measurement uncertainty. Repeatability can be strongly affected by external factors such as the installation location and the implementation.
Measurement uncertainty: Measurement uncertainty is defined as the sum of all error sources and the probability of adherence to the tolerance limits.
Minimum weight: The minimum weight is the minimum amount that can be weighed to achieve a defined measurement uncertainty.
Weighing range: The weighing range can extend from the minimum weight to the maximum load.
Acquisition time: The acquisition time (settling time) refers to the time that passes between the weight being placed on the balance and the measurement value appearing on the display.
USP requirements
Chapter <41> of the USP describes the requirements for balances with regard to repeatability, accuracy and the operating range.
Repeatability (USP <41>): The standard deviation is determined using 10 measurement values for one test weight. The repeatability is considered acceptable if two times the standard deviation divided by the target value of the weight used does not exceed 0.10%. If the standard deviation is less than 0.41 d (e. g. 0.041 mg at 0.1 mg readability) the standard deviation is replaced with 0.41 d (d = scale interval, i.e. the smallest difference that can be displayed).
Accuracy (USP <41>): The accuracy of a balance is satisfactory if the weighing value displayed (weighing carried out between 5% and 100% of the weighing range) is within 0.10% of the target weight value. Only test weights with a maximum calibration uncertainty of 0.03% may be used.
Operating range (USP <41>): The operating range has an upper limit that is determined by the maximum capacity of the balance and begins at the point at which the balance’s repeatability is ≤0.10%.
Calibration
"Measuring, weighing, recording and control equipment should be calibrated and checked at defined intervals by appropriate methods. Adequate records of such tests should be maintained."
Because weighing has a direct impact on the quantitative composition of the product, these controls must be carried out frequently. Official calibration of balances that are used for weighing in Production is not legally required if the measurement accuracy is ensured by carrying out regular calibration. However, it can make sense to have the balances in the weighing area officially calibrated with a view to ensuring a robust process is in place when handling potential complaints by the supplier in relation to the underfilling of containers.
Balance checks are usually carried out on a daily basis. In the weighing area, certified weights are weighed to check the defined measurement uncertainty. In addition, regular calibration across the entire operating range is carried out, e.g. on a monthly basis.
The eccentricity – i.e. the dependence of the measurement result on the position of the weight on the balance – must be checked on a regular basis. The test intervals can also be shortened here based on risk and available data, e. g. monthly or quarterly. The test frequency defined depends on the risk posed by possible deviations during testing.
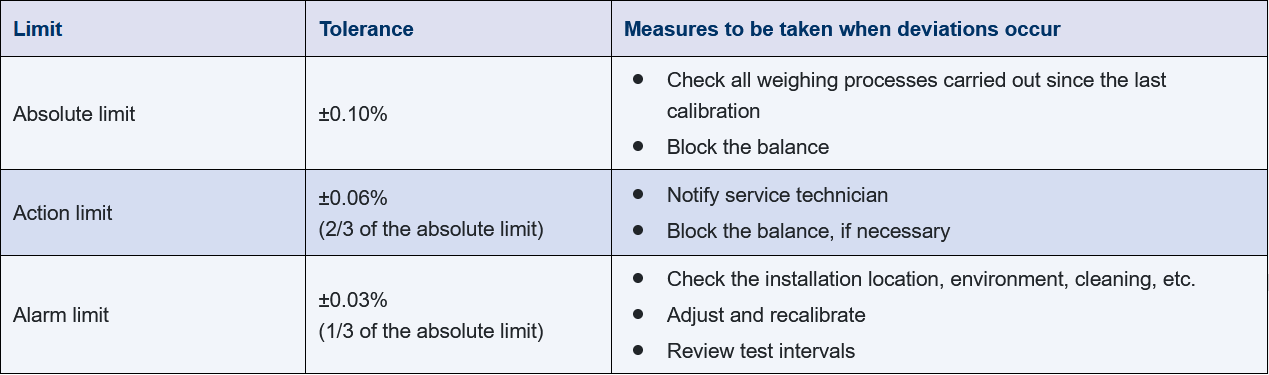
Usually, certified weights from class E2 to F2 or M are used for the tests depending on the weighing range and the balance. For the specification, absolute limits as well as warning and action limits can be defined that are evaluated using control charts, for example (see Figure 11.B-17). The measures required depend on the type of deviation. Violation of the absolute limit represents the worse case. If it happens, the balance must be taken out of operation. Furthermore, all of the weighing processes carried out since the last successful calibration must be checked (out of calibration).
Figure 11.B-17 Example: definition of limits during calibration
The tests must be documented accordingly. The weights used must be identified to ensure traceability.
It should be described how to handle the weights used for calibration. The aspects include:
- Labelling
- Storage and transport (protected)
- Cleaning (tools), disinfection or sterilisation, if applicable
- Placement of weights on balance (gloves, tweezers, etc.)
- Calibration frequency of these weights
During the calibration using higher-class weights, how the balances in the company will be checked must be defined.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








