
EMA Guideline on the Quality of Water for Pharmaceutical Use
Excerpt from "gmp review".
12 min. reading time | by Tim Sandle
Published in LOGFILE 07/2021
The 2020 EMA Guideline on the quality of water for pharmaceutical use deals with both purified water and water for injections (WFI). The document has been produced partly in relation to the change to the regulatory landscape in terms of producing WFI, where both distillation and reverse osmosis methods are permissible.
EMA raises concerns about endotoxin in relation to the reverse osmosis process. Today’s feature considers how the objectives for achieving chemical purity can be met, as well as general bioburden control measures.
Controlling water quality in pharmaceutical facilities requires an assessment of chemical and microbial risks. Of foremost concern is water-for-injections (WFI). This grade of water can be prepared using either reverse osmosis or by distillation.
Concerns with endotoxin risks in relation to reverse osmosis are central to a new European Medicines Agency guidance paper relating to pharmaceutical water production. This article considers the key messages within the guidance and the important learning points in relation to contamination control.
Introduction
The European Medicines Agency has produced the final version of its guideline ‘Guideline on the quality of water for pharmaceutical use’1. The document addresses purified water and WFI. The water source of greatest impact is WFI, because this is used as an ingredient water. This grade of water is also supplied for cleaning of product contact equipment and components, and it is the water supply to autoclaves in the form of steam. Purified water is used for equipment processing; it is supplied to laundries, used for hand washing, and as the source water for distillation.
The objectives of water purification are three-fold2.
- To reduce the levels of the chemical components in the water to prevent interactions with the drug substance, and to prevent toxicity to the patient. Toxicity is possible when large volumes are either infused or used in conjunction with dialysis.
- To reduce the microbial bioburden to the specified levels and to prevent further proliferation.
- To remove endotoxins and to prevent their future accumulation.
This article looks at the main points contained in the document in relation to using water of the appropriate quality for pharmaceutical manufacturing. The primary risk considerations concern microbial contamination, including bacterial endotoxin.
New guidance
The issuing of the new guidance, in July 2020, follows an earlier draft (with the same title) which was published in 2018. The draft remained out for public comment until mid-May 2019. As with any new guidance, this does not come into immediate effect and the document becomes part of the European Union Guidelines to Good Manufacturing Practice (EU Guidelines to GMP) on 1 February 2021. The primary change between the final version and the previous draft is the addition of an assessment of water quality for the preparation of herbal extracts (where the water quality is cross-referenced to the purified water standard).
Water is essential to pharmaceutical processing: present as an excipient; used for reconstitution of products; during synthesis; during production of the finished product; as a cleaning agent for rinsing vessels, equipment, primary packaging materials; and for the dilution of disinfectants. Therefore, water requires chemical and microbiological control commensurate with its intended application.
The purpose of the guidance is to set out the water quality requirements for specific pharmaceutical production processes (human and veterinary medicinal products, and advanced therapy medicinal products (ATMPs)). This relates to three different pharmaceutical water qualities: WFI, purified water and water for the production of extracts. The quality of mains (supply) water is outlined, although this is not considered to be water of pharmaceutical quality.
Water systems can become contaminated where the supply water is high in bioburden and this exceeds the design tolerances for the water purification process. The degree of bioburden reduction required varies according to the quality of the starting water and with seasonality. Variation is a factor of the complexity in community assembly, water matrices, physical structures, longitudinal land temporal dynamics, bio-geographical distributions, and chemical gradients from source to the pharmaceutical facility.
Water systems can also become contaminated where the water purification process is not operated to design parameters, such as through not operating membrane filtration systems or distillation units correctly. Hence, a suitably controlled means of preparation, storage and distribution must be employed to ensure that the limits are complied with at point of use.
One of the main reasons for the guidance being issued is to provide a regulatory perspective on the production of WFI using methods other than distillation, as per the earlier revision in the European Pharmacopoeia. On 1 April 2017, the European Pharmacopoeia monograph ‘Water for Injections’ (0169) was revised (in supplement 9.1) to allow for alternative methods, such as by reverse osmosis.
Reverse osmosis units use a semi-permeable membrane and a substantial pressure differential to drive the water through the membrane to achieve chemical, microbial and endotoxin quality improvements. The systems exist in multiple design formats and are often used in series. However, all reverse osmosis functions use a size-excluding filter operating under a highly pressurised condition. It will block 99.5% of endotoxin and ions/salts, but allow water molecules through.
With the more established method of distillation, this functions by turning water from a liquid to a vapour and then from vapour back to liquid. Endotoxin is removed by the rapid boiling which causes the water molecules to evaporate and the relatively larger endotoxin complex molecules to remain behind. Most models of distillation equipment are validated to achieve 2.5–3 log reductions in endotoxin concentration during distillation.
Under the European regulations, WFI is produced by either of the following.
- Distillation using an apparatus of which the parts in contact with the water are of neutral glass, quartz or a suitable metal and which is fitted with an effective device to prevent the entrainment of droplets.
- A purification process that is equivalent to distillation. Reverse osmosis, which may be single-pass or double-pass, coupled with other appropriate techniques such as electrodeionisation, ultrafiltration or nanofiltration, is suitable. Notice is given to the supervisory authority of the manufacturer before implementation.
This change means that the European Pharmacopoeia (and with it the European regulatory position) has become more closely aligned with the United States Pharmacopeia and the Japanese Pharmacopeia in terms of WFI generation. However, the inclusion of reverse osmosis as a water generation method has raised concerns in some quarters due to the risk in relation to bacterial endotoxin, as a biofilm could develop on the filter membrane (and biofilms are very difficult to eliminate once permanent microbial attachment has taken place). These risks relating to water produced by alternative methods are set out in an inspectorate working group document, where it is noted that reverse osmosis systems typically operate at ambient temperatures and as such offer an ideal environment for the formation of a biofilm3.
Biofilms and endotoxin
The central concern with biofilm build-up on the filter membrane is the risk of bacterial endotoxin passing through the filter membrane. Microbial biofilms develop when microorganisms adhere to a surface by producing extracellular polymers that facilitate adhesion and provide a structural matrix (or, to put it more crudely, slime-like structures develop). Microbial adherence is a consequence of the balance of attractive and repulsive physicochemical interactions between bacteria and the surface. Whilst the majority of bacteria are trapped within a biofilm, the biofilm will constantly generate bacteria that are released as free-floating individual cells and parts of the biofilm may slough off in clumps. The concern is such that as water is used and flows through the pipework or tap containing the biofilm, then the contamination risk arises at the point at which the water is used4.
To avoid the possibility of biofilm formation, the distribution system for water needs to be appropriately designed. This requires smooth internal surfaces in tanks and pipework. This is because microorganisms adhere less well to smooth surfaces than to rough surfaces. Care also needs to be paid to pipe joints and welds, as this can disrupt the surface smoothness. A further mechanism of importance is with the continuous movement of the water in tanks and the rapid flow of water within pipework. Where water is moving sufficiently rapidly and with the right level of turbulence, then shear forces mean that microorganisms will adhere poorly to surfaces. In contrast, where there is slow movement of water, microbes can begin to adhere to surfaces (hence the avoidance of areas where water can remain stagnant, such as ‘dead-legs’, is a key design principle). Water can also remain stagnant in valves, particularly at user points and even more particularly at user points which are not in frequent and regular use. This is counteracted by use of so-called hygienic or “zero dead-leg” valves.
Endotoxin is a component of the outer cell membrane of Gram-negative bacteria. Naturally occurring endotoxin is a complex containing cell wall components like phospholipids, lipoproteins and lipopolysaccharides. One part of lipopolysaccharides is called Lipid A, and it is this component that can stimulate the mammalian immune system, triggering a pyrogenic response (fever) or endotoxic shock. It is for this reason that endotoxin control of water systems is of paramount importance in relation to the manufacture of sterile medicines, especially those that are administered intravenously. For a pyrogenic response to be triggered, there needs to be large quantities of endotoxin within the blood stream (endotoxemia), derived from high numbers of Gram-negative bacteria.
Environmental endotoxin-produced Gram-negative bacteria in water is highly heterogeneous. The potency varies according to bacterial species and strain; and by solubility and molecular weight. The more potent endotoxins are those of the highest molecular Lipid-A weight and those which are most disaggregated. In water, endotoxin has a tendency to aggregate to form vesicles (membranous structures). The size of these vesicles is dependent upon the type of lipopolysaccharide structure and the pH, salt concentration and purity of the water. In pure water, the size is typically between 20,000 to 100,000 Daltons. Such environmental aggregates of endotoxin have a high affinity to surfaces5.
Key points from the guidance document
The main points from the guidance are outlined in Table 1. Readers should note that the table contains the key points; however, there are other aspects of water use that are outlined in the document.
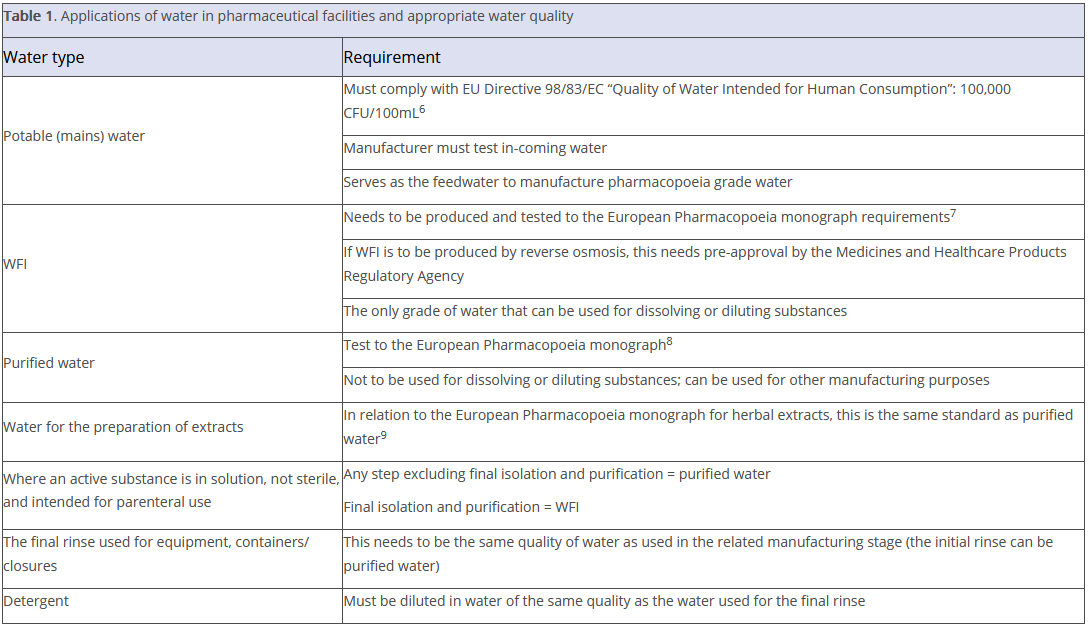
In relation to specific pharmaceutical products, the guidance indicates that the following is required in terms of water as an ‘active ingredient’.
- Biologics (including vaccines and ATMP): WFI.
- Parenteral: WFI.
- Ophthalmic: purified water.
- Haemofiltration solutions and haemodiafiltration solutions: WFI.
- Peritoneal dialysis solutions: WFI.
- Irrigation solutions: WFI.
- Nasal/ear preparations: purified water.
- Cutaneous preparation: purified water.
- All non-sterile products: purified water.
Furthermore, the document provides guidance in relation to the quality of water required for specific product types.
- Granulation: purified water.
- Tablet coating: purified water.
- Used in formulation prior to non-sterile lyophilisation: purified water.
- Used in formulation prior to sterile lyophilisation: WFI.
Such information provides clearer expectations as to the appropriate water quality for different manufacturing stages, much like the EU Guidelines to GMP Annex 1 provides examples of cleanroom activities against different cleanroom grades.
Summary
The new guidance document is useful, especially in providing examples of different applications of pharmaceutical water and the appropriate quality standards. The guidance supports the requirements of the European Pharmacopoeia and EU Guidelines to GMP, as well as providing an indication of the types of areas likely to be examined during a European regulatory inspection.
References
- European Medicines Agency. Guideline on the Quality of Water for Pharmaceutical Use. EMA/CHMP/CVMP/QWP/496873/2018. Amsterdam, The Netherlands: EMA; 20 July 2020. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-water-pharmaceutical-use_en.pdf - GMP Compliance Adviser, Chapter C.11
- Sandle T. An approach for the reporting of microbiological results from water systems. PDA Journal of Pharmaceutical Science and Technology 2004;58(4):231–237.
- GMP/GDP Inspectors Working Group. Questions and Answers on Production of Water for Injections by Non-Distillation Methods – Reverse Osmosis and Biofilms and Control Strategies EMA/INS/GMP/443117/2017. Amsterdam, The Netherlands: EMA; 1 August 2017. Available at: https://www.ema.europa.eu/en/documents/other/questions-answers-production-water-injections-non-distillation-methods-reverse-osmosis-biofilms_en.pdf
- Sandle T. Bacterial adhesion: an introduction. Journal of Validation Technology 2013;19(2). Available at: http://www.ivtnetwork.com/article/bacterial-adhesion-introduction
- Sandle T. Bacterial endotoxin testing using the limulus amebocyte lysate assay. In: Kőszegi T and Chesca A (Eds), Laboratory Techniques with Applicability in Medical Practice. Rīgā, Latvia: Lambert Academic Publishing; 2015, pp. 19–32.
- Legislation.gov.uk. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available at: https://www.legislation.gov.uk/eudr/1998/83
- European Directorate for the Quality of Medicines. European Pharmacopoeia, “Water for Injections” (0169). Strasbourg, France: EDQM, 1 April 2017.
- European Directorate for the Quality of Medicines. European Pharmacopoeia, “Water Purified” (0008). Strasbourg, France: EDQM.
- European Directorate for the Quality of Medicines. European Pharmacopoeia, “Water for Preparation of Extracts” (2249). Strasbourg, France: EDQM.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







