
Thoughts on the transport of pharmaceuticals from a claims handling perspective
13 min. reading time | by Axel Radke
Published in LOGFILE 41/2020
Research, development and production of a medicinal product are usually in the hands of the marketing authorization holder (MAH). These processes are therefore subject to the direct supervision and control of the MAH.
However, the picture is different for transport to the customer or to the worldwide subsidiaries and commercial agencies. 90% of the manufacturers hand over their medicines into the hands of logistics service providers and thus into an area that often escapes direct control. However, the EU GMP Guidelines also bind the manufacturer to his responsibility for the products. In international transport this quickly becomes a major challenge.
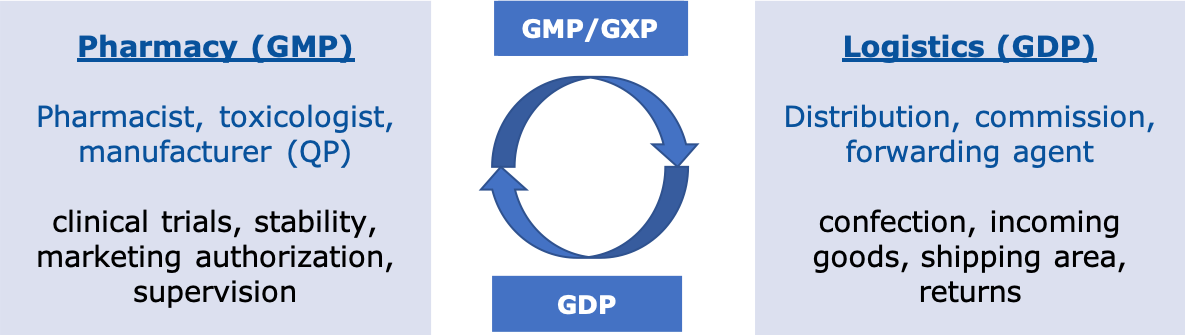
Figure 1: Interaction shipper – logistician (Source: Axel Radke)
It only works together - Good!
Wednesday 15.00 - email receipt at the forwarding agency:
Sender: Pill Twister L
Wednesday 15.00 - email receipt at the forwarding agency:
Sender: Pill Twister Ltd.
Recipient: Pill Twister Inc.
Quantity: 20 pallets
Can you get this cheaply into the USA?
Unfortunately, in daily practice one still encounters the situation that consignments of highly sensitive pharmaceutical products are registered for transport with any forwarding agent, with the only question being how to keep costs to a minimum. The notification of the transport is usually made by email - short and concise, often with only a few details about the scope of the shipment and the recipient. After the consignment has been handed over to the forwarding agent, it is assumed that everything will go its right way. But this way leads all too often directly to the total loss of the medicines!
Already in the selection of partners in transport, the EU GDPs‘ questions of qualification and validation, i.e. the examination of suitability for the intended transport route, must be in the foreground. Only service providers whose suitability has been tested and proven can ultimately provide a GDP compliant service for the transport of pharmaceuticals. However, simply working through an audit catalogue is not enough. Rather, a trustworthy and constructive partnership should already be established in the planning phase and during the drafting of the logistics contracts, which then continues in the daily cooperation and extensive flow of information. Everyone here is the expert and advisor of the other in his or her own field and therefore it only works together - good.
Editorial tip:
Read in the GMP Compliance Adviser what you have to pay attention to when choosing a service provider and how you can carry out a qualification (Chapter 16.P Logistics service providers).
Refugee flows - What do they have to do with GDP?
Tuesday morning 10:00: the shipment of medicinal products from Greece is expected urgently. But when the back doors are opened, a picture of destruction appears. The truck tarpaulin is slit at the top. All pallets are crushed, cartons are torn open and there is rubbish and dirt everywhere. It smells of urine. During further unloading, dirty paper towels and PE bottles filled with urine are found.
A link between migration to Europe and the transport of pharmaceuticals appears at first sight to be far-fetched. But everyday life in the world of transport shows something else: we too must deal with this issue! The topic of "immigrants" must be taken into account as early as the planning of transport routes, rest areas and the selection of vehicles and technical equipment. Everyone is talking about the French port of Calais, with hundreds of immigrants who want to gain access to trucks and thus reach Great Britain. But Calais is no longer an isolated case. Rather, in recent years we have seen an increase in the number of "rides" on trucks throughout Europe, especially on routes from the south and south-east. Once the immigrants have travelled along on the load for several days without being noticed, a total loss of the medicines can hardly be avoided.


Figures 2 & 3: Human excrement and waste on and between the load (Source: Axel Radke)
New Europe - long ways
Friday 13:30 call from the forwarding agency: "Our truck has been standing at the border crossing for 8 hours and the waiting time will probably be another 4-5 hours. Delivery this afternoon will not work. We will then deliver Monday morning." The sender is in a hurry, because the thermal shipping box, packed for 48 hours, cannot cope.
The almost habitual dream of a Europe without frontiers with a rapid movement of goods has taken on clear shadows due to the political changes of the last two years. New border and import controls have led to long waiting times and to a significant increase in transport times, especially for land transport by truck. Problems with short shelf-lives or the lead times of passive temperature control in thermal shipping boxes are examples of this. Transport planning must also be set up and adapted to such situations. Marginal days before weekends or public holidays should be avoided and generous temperature reserves should be taken into account.
Editorial tip:
Read in the GMP Compliance Adviser how to identify, assess and control transport risks and to what extent transport validation/verification is possible (Chapter 16.M Risk management in transportaition and Chapter 16.Q Transport validation).
New risk - climate change
Monday 13:00 in July 2018: the sun is laughing from the sky, not a cloud in sight, 45°C above the concrete: hectic at the airport, the departure of the cargo plane to Dubai is imminent. The air freight pallets are prepacked, freight boxes are stowed and the first units are already being taken to the runway. Among other things, refrigerated medicines are also being transported. The consignment is parked on the tarmac, but refuelling is delayed and the loading stops.
The experiences from the summer of 2018 have clearly shown us that the climatic changes also play a role that should not be underestimated in the planning and execution of pharmaceutical transports. Where we previously had moderate temperatures (+25°C), maximum values above 40°C were now measured. On runways and concrete slabs, even long-term temperatures above 50°C were reached. Many an evaluation of the data loggers from the shipments then caused a nasty surprise.
Particularly when looking at air freight, it must be remembered that temperature-controlled consignments in the context of runway transport and aircraft loading are also exposed to these extreme temperatures and cannot always be completely cooled. Special services of refrigerated transports on the airfield or the use of Envirotainers must be ordered in advance and included in the freight costs.
Previously common reloading or loading without temperature-controlled loading ramps must be checked. Storage in halls up to +25°C without temperature control, which was possible up to now, can quickly become a pitfall in the summer months. The previous summer configuration of passively cooled thermal shipping boxes must also be checked and adapted to these new conditions in order to prevent damage to the medicines.
Editorial tip:
Read in the GMP Compliance Adviser how to qualify thermal shipping containers for different climate zones and which quality standards are to be applied for airfreight transport (Chapter 16.O Qualification of passive insulation boxes and Chapter 16.K Modes of transportation).
Final destination - mould infestation
After opening the sea freight container, the horror is written all over the faces of all those involved. Mould is growing from the pallets and the container smells disgraceful. More than 20 pallets of important medicines are no longer usable and the economic damage is enormous.
The formation of mould, mainly during transport in sea freight containers, can have many reasons. In particular, however, the introduction of moisture into the container as a result of the conditions during loading (high humidity at the place of loading) or damp cardboard and wooden pallets is a recurring problem. The circumstances under which these packaging materials are stored and also their manufacture play an important role. In the context of procurement, constant control and monitoring is therefore important. Particularly due to the very strong increase in demand for wooden pallets, there is a risk of decreasing quality in terms of wood quality and sufficient drying. For this reason, the condition and moisture content (<20%) of the wood should be checked at least in random samples on receipt of the goods, as well as during subsequent use. For own storage, well ventilated and dry areas should also be used.

Figure 4: Mould on pallets (Source: Axel Radke)
Shipping by parcel service - a mechanical challenge
The doorbell rings and in front of us stands the delivery man from the parcel service. He hands over two small parcels with medicines. As our order was very small this time, it was probably not worthwhile to transport them with the forwarding agency and a direct courier service would have been far too expensive. One parcel seems to be a bit damp and is bound in foil. For the other parcel the cardboard is quite ok. We sign on the pad and let the parcel carrier go.
But when we open the parcels we are a little surprised:


Figures 5 & 6: Damage for parcel service shipments (Source: Axel Radke)
The use of the usual parcel services with their fixed, national and international rates is gladly taken up for smaller shipments. In addition, their services are very convenient: the goods are collected on site and delivered directly to the customer during normal business hours.
If these services are used, however, one should also be aware that the usual handling of parcels in daily bulk business involves an enormous mechanical stress for the packaging and the contents. Here the parcels pass through many handling points, are transported in automatic sorting and conveyor systems with roller conveyors and chutes and are tightly stowed in the vehicles. Pressure, impact and shock are the usual stresses to which the packaging, but especially the contents, must be able to withstand. Moreover, GDP compliant temperature control is not possible with normal parcel services. On closer inspection, the sensitive pharmaceutical products are therefore often unsuitable for normal parcel services. Especially containers with liquids or glass containers are very problematic here. However, many parcel services now offer a special service for pharmaceutical transports, which are then also possible in the temperature-controlled area. However, these services must already be ordered or requested as part of the transport order.
Editorial tip:
Read more about the advantages and disadvantages of different distribution routes in the GMP Compliance Adviser (Chapter 16.P.3 Different distribution routes and methods: Critical points, taking cold chain logistics as an example).
Use of thermal shipping boxes
Quickly take the carton from the shelf, insert the polystyrene sheets and put the dry ice on top. Afterwards everything is well glued and the shipping label is stuck on the box. Ready for dispatch - or not?
Admittedly: the thermo shipping box in the self-construction is meanwhile probably rather the exception - but unfortunately still occasionally to be found in the shipping department. We clearly advise against such experiments or inexpensive variants. But even with commercial shipping boxes, it happens again and again that the products have been damaged by the dry ice or that the cooling has already been suspended during transport. In these cases, the correct configuration of the shipping boxes was usually missing. A validated packing scheme for the different components of product, packaging and dry ice/cooling batteries, as required by the EU GDPs and the implementation guidelines, was missed. However, the correct functioning of the thermal shipping boxes and thus the undamaged transport of the medicines is dependent on a validated and documented packing scheme. During validation, the different seasons or shipping routes must also be taken into account. The boxes are offered by different manufacturers and made of different materials. Despite the technical specifications regarding performance (temperature holding capacity), it must be remembered that the final validation on the own products and applications can only be performed and documented by the shipper.
Figure 7: Thermal shipping box: Does my configuration still meet the climatic conditions? (Source: GMP Compliance Adviser)Abbildung 7: Thermoversandbox: Entspricht meine Konfiguration noch den klimatischen Bedingungen? (Quelle: GMP-BERATER, GMP-Verlag)
Editorial tip:
Read in the GMP Compliance Adviser which criteria are important for the selection of thermal shipping boxes and how to carry out the qualification correctly (Chapter 16.O Qualification of passive insulation boxes).
Data logger - Helpful addition or too expensive?
Example 1:
The dispatch manager has just received a hectic call from the forwarding agent. On the temperature-controlled transport to Italy the recording of the temperature control has failed for several hours and the alarm has not been triggered. Now the recipient cannot be presented with a complete record of the temperature control and the quality assurance department wants to destroy the pharmaceutical shipment immediately.
As a matter of principle, a complete proof of the required storage temperature is necessary for the life cycle of pharmaceuticals and thus also for the phase of transport. Deviations and uncertainties therefore often lead by law to total loss and high monetary burdens. Various telematics systems with and without GPS or GSM monitoring are available today for monitoring temperatures in trucks. Despite regular maintenance and technical upgrades, however, even the best technology can fail at some point - and with it the verification of the temperature curve. In many cases, common sense dictates that the temperature of the products loaded in the trailer must be kept within the specified temperature range. However, the proof is now missing.
Therefore, the question is allowed here why additional loggers were not used in the consignment, which can provide further information about the actual temperature profile of the products. The use of the loggers can thus help to avoid a total loss. Often this question refers to the additional effort and costs. In view of the consequences, however, this answer is difficult to understand and should definitely be questioned.
Example 2:
Email from our customer in the Far East: The shipment of 30 pallets of medicines (2 to 8°C) arrived yesterday as planned. However, in the incoming goods department it was discovered that the 2 data loggers show a temperature peak of +19°C over 3 hours. It is assumed that all pallets were too warm and the customer does not want the goods anymore.
Unfortunately, we often encounter this case in claims processing. In the case of a shipment with various packages, only a few of the packages are fitted with a data logger. In the case of a discrepancy, it is then assumed that the entire shipment was affected, but the proof is completely missing. Especially when handling in transshipment at airports, it is not uncommon for shipments to be temporarily split or distributed over several air freight pallets. The conclusion of a deviation from one to all packages is therefore difficult in the damage assessment and is often doubted due to the lack of proof. In addition, the question must also be allowed here whether a high monetary loss and the trouble with the customer could have been avoided by using additional data loggers. Here again, the commercial argument of the high costs for the data loggers is often used. But is this expenditure really so high? The answer can only be found by each shipper on his own.
Editorial tip:
In the GMP Compliance Adviser you will find all the important information on temperature monitoring during transport using data loggers and telematics systems (Chapter 16.L Monitoring).
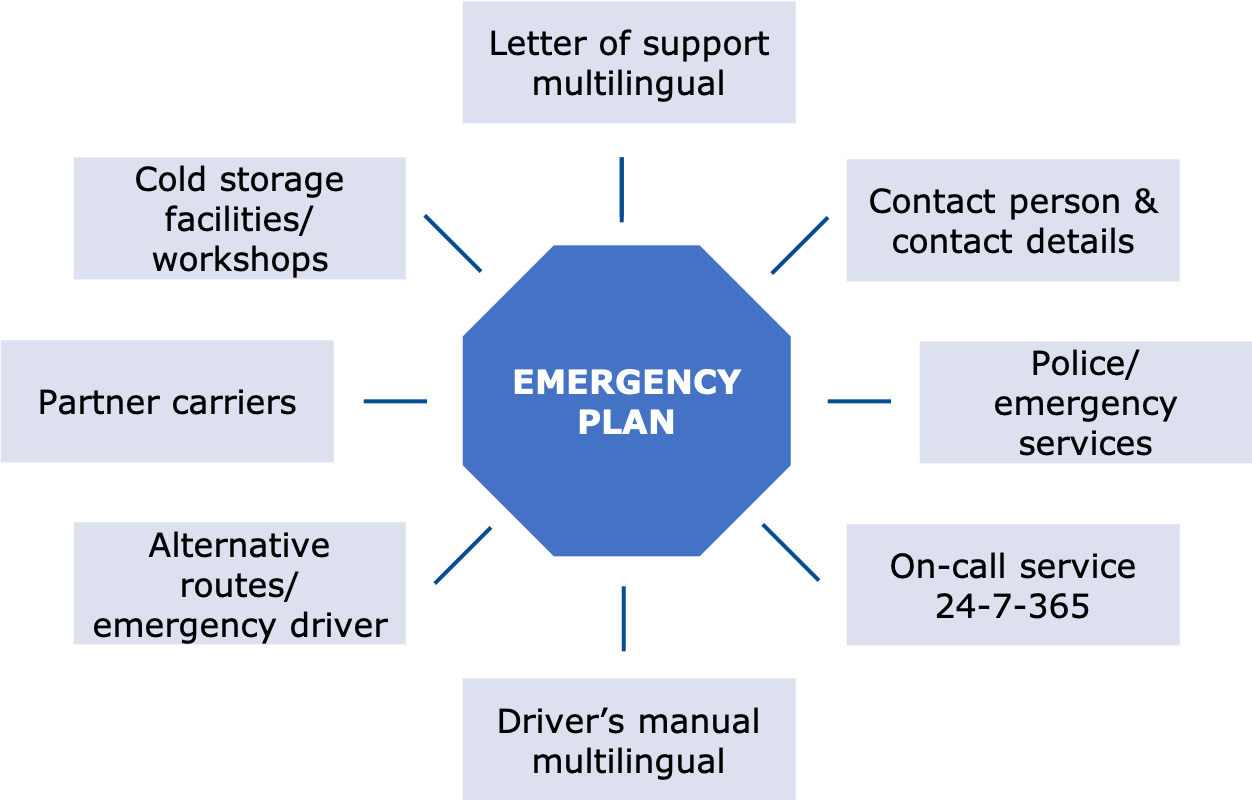
Emergency plan - Have you thought about it?
Actually, it is human to prefer to ignore the possible occurrence of emergencies and live according to the motto: "It will be alright!“ In the context of pharmaceutical logistics, however, it is important to think about such incidents and to have an intensive exchange of information with the service provider about emergency scenarios and emergency plans. Many damages could have been avoided or at least would not have ended in a total loss if correspondingly effective emergency procedures had been established and tested. The plans are not only to be stored in the joint documentation (SOP), but are also to be made available to the driver in the form of a leaflet or app with the consignment. This closes the circle of the close cooperation with the service provider already described above.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







