
Trust is good, calibration is better!
Shortened excerpt from the GMP Compliance Adviser, Chapter 4.F Calibration
11 min. reading time | by Hansjörg Gutmann, Markus Kopf, Markus Salemink, Christian Sander, PhD
Published in LOGFILE 10/2020
Numbers play a key role in the manufacture of medicinal products. From the receipt of the raw materials to the final release of the finished product, results (actual values) are repeatedly compared with specifications (target values) to decide whether the quality meets the requirements. This applies equally to measured values in the manufacturing process and to analytical results in the laboratory.
Measured values therefore decide on the acceptance or rejection of raw materials, intermediates and finished products. Measured values must be reliable:
Only correct (measured) values lead to correct decisions.
Therefore, in the pharmaceutical industry, the entirety of all quality-relevant metrological equipment must be calibrated. Metrology is relevant to quality, when we make decisions based on the results.
What does calibration mean?
Official definition
Calibration: Operation that, under specified conditions, in a first step, establishes a relation between the quantity values with measurement uncertainties provided by measurement standards and corresponding indications with associated measurement uncertainties and, in a second step, uses this information to establish a relation for obtaining a measurement result from an indication.
Source: JCGM 200:2012; International vocabulary of metrology – Basic and general concepts and associated terms (VIM)
Simplified Explanation
Calibration: Calibration is a procedure whereby the measurement value is compared with the correct value of a standard at predetermined conditions. Calibration also includes the documentation of deviations, the calculation of measurement uncertainty and the provision of a certificate.
Why is calibration so important?
Calibration leads to reliability and comparability
Calibration makes it possible to obtain reliable measurement results. Reliable measurement results, in turn, allow comparisons to be made. Calibration leads to reliability and comparability:
- Correct measurement results help us to make objective decisions that lead to greater certainty.
- Based on the calibration results and the measurement uncertainty, we can make quantitative statements about realistic accuracies. Is my instrumentation able to provide sufficiently accurate results for the intended application?
- Metrological traceability to national and international standards ensures worldwide comparability. For example, a 5°C storage temperature in Germany corresponds to a 5°C storage temperature in Japan.
- When transferring production plants or manufacturing processes to other locations, calibration helps to provide globally reproducible measurement results and thus supports the robustness of processes.
Calibration is retrospective
During calibration, only the measurement accuracy of the instrument at the time of calibration is determined. Strictly speaking, no statements can be made about the measurement results in the past or in the future.
However, historical data can be used with a certain degree of probability to determine whether the instrument has delivered correct results in the past and it is even possible to make predictions about future behaviour.
During calibration, we verify that between two calibration events the measurement results of the individual instrument are obtained in accordance with the specifications.
|
“ Calibration is always looking at the past performance ” Simply stated: |
| Did my instrument provide correct measurement results in the pe-riod between the previous and the current calibration? |
Calibration thus provides an important evaluation factor for making decisions regarding metrologically induced statements.
This evaluation factor can, of course, have far-reaching effects if, for example, process parameters are incorrectly controlled or release testing is performed with out-of-tolerance test equipment.
Calibration is a regulatory requirement
In additional to the logical sense of performing calibrations, regulatory requirements also govern the practice. EU GMP Guidelines Part I, Chapter 3.41 stipulates:
“Measuring, weighing, recording and control equipment should be calibrated and checked at defined intervals by appropriate methods. Adequate records of such tests should be maintained.”
Regulatory requirements for calibration are also found in 21 CFR Part 211 (§§ 68 a and 160 b) and in Part 820 § 72a. DIN EN ISO 13485 and ISO 9001:2015 also require the calibration of measuring equipment.
Which calibration procedures exist?
It is possible to perform calibrations either by direct exposure to the conditions at a quantity setting or by performing comparison measurements. In general, the method is selected based on the effort required and the ability to meet the technical requirements in relation to the required level of precision.
Direct exposure
When calibrating by direct exposure, auxiliary equipment is used to “apply” specific measurement quantity values to the device being calibrated as well as the reference.
An example of such auxiliary equipment is a tempered water bath. The temperature probe of the device being calibrated and the higher precision reference probe are both inserted into the bath. The probes are “exposed” to various calibration points by varying the temperature of the liquid (heating and cooling).
A multi-point calibration or calibration using at least one specific point requires that these values are applied to the test object and the reference. For this reason, multi-point calibrations are generally performed by direct exposure. A typical test set-up is shown in Figure 1.
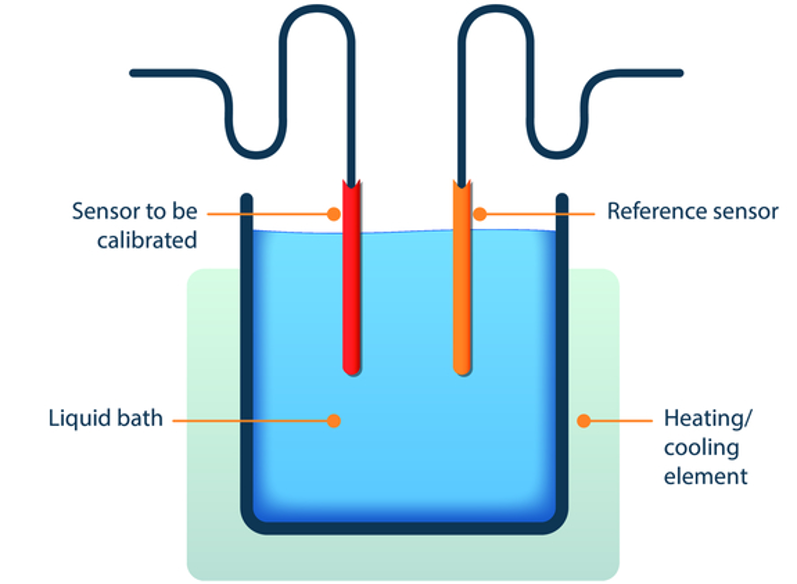
Figure 1 Calibration of a temperature probe by direct exposure in a tempered bath (courtesy of Testo Industrial Services)
Comparative measurement
The most common form of a comparative measurement is a comparison at the working point. With such a calibration, no previously defined value for exposure of the reference and test instrument is specified; instead the value already prevailing in the test environment is accepted as the calibration point. For example, an air temperature sensor that records the room temperature in control mode remains installed at its location. It also records the room temperature, which is 20°C, for example. The calibration is now carried out in such a way that a reference sensor is brought as close as possible to the test object (e.g. the air temperature sensor in the room). Then the target/actual comparison takes place.
A comparative measurement is recommended in cases where it is not possible to remove a sensor from its location in the process or if the recording of the parameters of the running process should not be interrupted for calibration.
A typical test measurement set-up for a comparator measurement is shown in Figure 2.
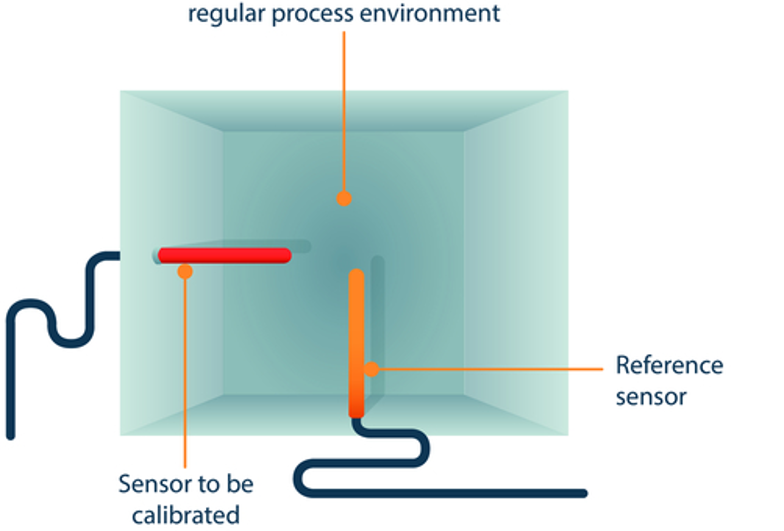
Figure 2 Calibration of a room temperature sensor by comparator measurement at the operating point (courtesy of Testo Industrial Services)
Measurement chain
A measurement chain comprises the instruments and auxiliary equipment which together are involved in displaying the measurement signal.
Generally, measurement chains are used to normalise different output variables to a uniform measurement signal. This enables signal transmission to a central processing and control unit. Each sensor within this measuring chain converts a measured variable recorded as an input signal into a corresponding output signal. This is illustrated schematically in Figure 3.
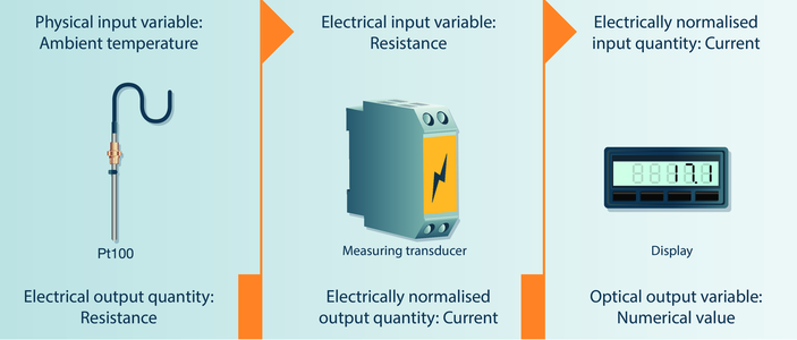
Figure 3 Example of a measurement chain (courtesy of Testo Industrial Services)
Electrical simulation
Electrical simulation becomes necessary when the calibration of a measurement chain as an interconnected unit cannot be performed.
If, for example, only the display shown in the example of a measurement chain shown above is to be calibrated, the input signal needs to be simulated. The display device is then exposed to different current values by in electronic calibrator. The calibration is then performed by comparison of the expected display values corresponding to the different current levels with the actual displayed values.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de








