
Packaging material testing
5 min. reading time | by
André Deister, Sabine Mendel
Published in LOGFILE 06/2020
Pharmaceutical packaging materials have a variety of functions. The main purpose of primary packaging materials, for example, is to protect the medicinal product against the physical effects of light, air and moisture. Secondary packaging materials provide information on the amount, dosage type and application of the packaged medicinal product.
However, secondary packaging, in particular, is increasingly being used to fulfil other functions such as the prevention of counterfeiting. It is also used for marketing purposes, to ensure child safety and for braille text. The wide range of packaging material functions is reflected in the complex testing of packaging material components.
Packaging material suppliers
The supplier of the packaging materials plays an important role when pharmaceutical packaging materials undergo testing. Because of the numerous materials involved, the complexity of the manufacturing process and the extremely specific test parameters, many of the packaging material tests are carried out at the supplier.
The pharmaceutical company must be able to prove that the packaging material components provided by the supplier have undergone proper testing and comply with the specifications.
To ensure that this is the case, it is absolutely essential to reach written agreement on the following in advance:
- type and scope of the supplier certificate
- type and number of the random samples provided
- definition of a delivered batch
- definition of responsibilities (QA agreement)
In the case of packaging materials that have to fulfil special requirements, a Certificate of Analysis (CoA) from the supplier can be extremely helpful. A CoA, unlike a Certificate of Conformity (CoC), contains concrete results. These can be evaluated by the pharmaceutical purchaser and adapted to its own evaluation system, if necessary. A CoC only confirms that the packaging materials comply with the requirements.
A practical tip: The CoA and data recorded by the supplier can be used to create trend analyses and show quality developments.
Quality Control is responsible for the incoming goods inspection of packaging material components which is often carried out using samples provided by the supplier. Because these samples are not taken randomly from the overall batch by the company's own QC, a written agreement governing the sample-taking process must be in place.
If continuous manufacturing is used to produce the packaging material components, it is important that agreement is reached with the supplier on how a batch is defined. A statistical sample quantity can only be calculated based on a clear batch definition.
This and other specifications are documented in QA agreements that outline the responsibilities in the commercial relationship between the supplier and pharmaceutical purchaser with regard to quality. The possible content of a QA agreement is summarised in Figure 1.
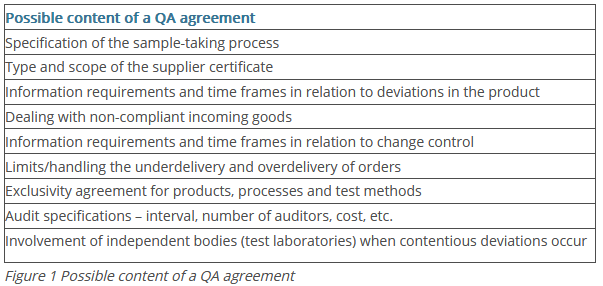
Agreement about these documents is often part of the supplier qualification. The supplier qualification is the responsibility of the QA department and is meant to ensure that the new supplier has established quality systems and manufacturing processes in place that are necessary for producing products that are consistent, safe and of a high quality.
The supplier qualification is carried out by auditing the supplier, and in exceptional cases, by sending written questionnaires to the supplier. A specific number of batches that is used to assess the degree of compliance with the quality requirements is often agreed with the supplier for this purpose. The results from these (qualification) batches are decisive for a positive qualification of the supplier.
Important: The initial (qualification) batches should be tested in full to verify compliance with the agreed specifications.
Do you have any questions or suggestions? Please contact us at: redaktion@gmp-verlag.de







